Regulation of dental enamel shape and hardness
- PMID: 20675598
- PMCID: PMC3086535
- DOI: 10.1177/0022034510375829
Regulation of dental enamel shape and hardness
Abstract
Epithelial-mesenchymal interactions guide tooth development through its early stages and establish the morphology of the dentin surface upon which enamel will be deposited. Starting with the onset of amelogenesis beneath the future cusp tips, the shape of the enamel layer covering the crown is determined by five growth parameters: the (1) appositional growth rate, (2) duration of appositional growth (at the cusp tip), (3) ameloblast extension rate, (4) duration of ameloblast extension, and (5) spreading rate of appositional termination. Appositional growth occurs at a mineralization front along the ameloblast distal membrane in which amorphous calcium phosphate (ACP) ribbons form and lengthen. The ACP ribbons convert into hydroxyapatite crystallites as the ribbons elongate. Appositional growth involves a secretory cycle that is reflected in a series of incremental lines. A potentially important function of enamel proteins is to ensure alignment of successive mineral increments on the tips of enamel ribbons deposited in the previous cycle, causing the crystallites to lengthen with each cycle. Enamel hardens in a maturation process that involves mineral deposition onto the sides of existing crystallites until they interlock with adjacent crystallites. Neutralization of acidity generated by hydroxyapatite formation is a key part of the mechanism. Here we review the growth parameters that determine the shape of the enamel crown as well as the mechanisms of enamel appositional growth and maturation.
Figures

 after infusion with labeled proline for 10 days, suggesting daily fluctuations in the secretion of collagen (Ohtsuka et al., 1998).
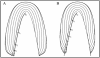
after infusion with labeled proline for 10 days, suggesting daily fluctuations in the secretion of collagen (Ohtsuka et al., 1998).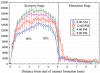






References
-
- Adeleke-Stainback P, Chen E, Collier P, Yuan ZA, Piddington R, Decker S, et al. (1995). Analysis of the regulatory region of the bovine X-chromosomal amelogenin gene. Connect Tissue Res 32:115-118 - PubMed
-
- Al Kawas S, Warshawsky H. (2008). Ultrastructure and composition of basement membrane separating mature ameloblasts from enamel. Arch Oral Biol 53:310-317 - PubMed
-
- Almaani N, Liu L, Dopping-Hepenstal PJ, Lovell PA, Lai-Cheong JE, Graham RM, et al. (2009). Autosomal dominant junctional epidermolysis bullosa. Br J Dermatol 160:1094-1097 - PubMed
-
- Aoba T, Moreno EC. (1987). The enamel fluid in the early secretory stage of porcine amelogenesis: chemical composition and saturation with respect to enamel mineral. Calcif Tissue Int 41:86-94 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- DE011301/DE/NIDCR NIH HHS/United States
- R56 DE011301/DE/NIDCR NIH HHS/United States
- R21 DE018878/DE/NIDCR NIH HHS/United States
- DE015846/DE/NIDCR NIH HHS/United States
- R01 DE019622/DE/NIDCR NIH HHS/United States
- DE019775/DE/NIDCR NIH HHS/United States
- R01 DE015846/DE/NIDCR NIH HHS/United States
- DE018878/DE/NIDCR NIH HHS/United States
- R01 DE011301/DE/NIDCR NIH HHS/United States
- R29 DE011301/DE/NIDCR NIH HHS/United States
- R56 DE015846/DE/NIDCR NIH HHS/United States
- R01 DE019775/DE/NIDCR NIH HHS/United States
- DE019622/DE/NIDCR NIH HHS/United States
LinkOut - more resources
Full Text Sources

