Polyembryony in non-apomictic citrus genotypes
- PMID: 20675656
- PMCID: PMC2944972
- DOI: 10.1093/aob/mcq148
Polyembryony in non-apomictic citrus genotypes
Abstract
Background and aims: Adventitious embryony from nucellar cells is the mechanism leading to apomixis in Citrus sp. However, singular cases of polyembryony have been reported in non-apomictic genotypes as a consequence of 2x × 4x hybridizations and in vitro culture of isolated nucelli. The origin of the plants arising from the aforementioned processes remains unclear.
Methods: The genetic structure (ploidy and allelic constitution with microsatellite markers) of plants obtained from polyembryonic seeds arising from 2x × 4x sexual hybridizations and those regenerated from nucellus culture in vitro was systematically analysed in different non-apomictic citrus genotypes. Histological studies were also conducted to try to identify the initiation process underlying polyembryony.
Key results: All plants obtained from the same undeveloped seed in 2x × 4x hybridizations resulted from cleavage of the original zygotic embryo. Also, the plants obtained from in vitro nucellus culture were recovered by somatic embryogenesis from cells that shared the same genotype as the zygotic embryos of the same seed.
Conclusions: It appears that in non-apomictic citrus genotypes, proembryos or embryogenic cells are formed by cleavage of the zygotic embryos and that the development of these adventitious embryos, normally hampered, can take place in vivo or in vitro as a result of two different mechanisms that prevent the dominance of the initial zygotic embryo.
Figures






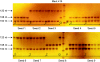
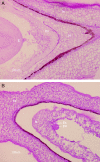
References
-
- Alleman M, Doctor J. Genomic imprinting in plants: observations and evolutionary implications. Plant Molecular Biology. 2000;43:147–161. - PubMed
-
- Bacchi O. Cytological observations in citrus III. Megaesporogenesis, fertilization and polyembryony. Botanical Gazette. 1943;105:221–225.
-
- Batygina TB. New approach to the system of reproduction in flowering plants. Phytomorphology. 1989;39:311–325.
-
- Batygina TB. Morphogenesis of somatic embryos developing in natural conditions. Biologija. 1998;3:61–64.

