Growth inhibition mediated by PSP94 or CRISP-3 is prostate cancer cell line specific
- PMID: 20676114
- PMCID: PMC3739318
- DOI: 10.1038/aja.2010.56
Growth inhibition mediated by PSP94 or CRISP-3 is prostate cancer cell line specific
Abstract
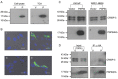
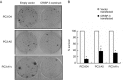
The prostate secretory protein of 94 amino acids (PSP94) has been shown to interact with cysteine-rich secretory protein 3 (CRISP-3) in human seminal plasma. Interestingly, PSP94 expression is reduced or lost in the majority of the prostate tumours, whereas CRISP-3 expression is upregulated in prostate cancer compared with normal prostate tissue. To obtain a better understanding of the individual roles these proteins have in prostate tumourigenesis and the functional relevance of their interaction, we ectopically expressed either PSP94 or CRISP-3 alone or PSP94 along with CRISP-3 in three prostate cell lines (PC3, WPE1-NB26 and LNCaP) and performed growth inhibition assays. Reverse transcription-polymerase chain reaction and Western blot analysis were used to screen prostate cell lines for PSP94 and CRISP-3 expression. Mammalian expression constructs for human PSP94 and CRISP-3 were also generated and the expression, localization and secretion of recombinant protein were assayed by transfection followed by Western blot analysis and immunofluorescence assay. The effect that ectopic expression of PSP94 or CRISP-3 had on cell growth was studied by clonogenic survival assay following transfection. To evaluate the effects of co-expression of the two proteins, stable clones of PC3 that expressed PSP94 were generated. They were subsequently transfected with a CRISP-3 expression construct and subjected to clonogenic survival assay. Our results showed that PSP94 and CRISP-3 could each induce growth inhibition in a cell line specific manner. Although the growth of CRISP-3-positive cell lines was inhibited by PSP94, growth inhibition mediated by CRISP-3 was not affected by the presence or absence of PSP94. This suggests that CRISP-3 may participate in PSP94-independent activities during prostate tumourigenesis.
Figures




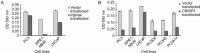
Comment in
-
Growth inhibition properties of the putative prostate cancer biomarkers PSP94 and CRISP-3.Asian J Androl. 2011 Mar;13(2):205-6. doi: 10.1038/aja.2010.120. Epub 2010 Nov 22. Asian J Androl. 2011. PMID: 21102472 Free PMC article. No abstract available.
Similar articles
-
Growth inhibition properties of the putative prostate cancer biomarkers PSP94 and CRISP-3.Asian J Androl. 2011 Mar;13(2):205-6. doi: 10.1038/aja.2010.120. Epub 2010 Nov 22. Asian J Androl. 2011. PMID: 21102472 Free PMC article. No abstract available.
-
Purification and characterization of CRISP-3 from human seminal plasma and its real-time binding kinetics with PSP94.J Chromatogr B Analyt Technol Biomed Life Sci. 2016 Dec 15;1039:59-65. doi: 10.1016/j.jchromb.2016.10.032. Epub 2016 Oct 26. J Chromatogr B Analyt Technol Biomed Life Sci. 2016. PMID: 27825912
-
Mapping of the binding sites involved in PSP94-CRISP-3 interaction by molecular dissection of the complex.Biochim Biophys Acta. 2013 Apr;1830(4):3019-29. doi: 10.1016/j.bbagen.2013.01.015. Epub 2013 Jan 31. Biochim Biophys Acta. 2013. PMID: 23375721
-
Structural and molecular biology of PSP94: Its significance in prostate pathophysiology.Front Biosci (Landmark Ed). 2018 Jan 1;23(3):535-562. doi: 10.2741/4604. Front Biosci (Landmark Ed). 2018. PMID: 28930560 Review.
-
Relevance of CRISP proteins for epididymal physiology, fertilization, and fertility.Andrology. 2019 Sep;7(5):610-617. doi: 10.1111/andr.12638. Epub 2019 Jun 19. Andrology. 2019. PMID: 31218833 Review.
Cited by
-
SRC kinase regulation in progressively invasive cancer.PLoS One. 2012;7(11):e48867. doi: 10.1371/journal.pone.0048867. Epub 2012 Nov 7. PLoS One. 2012. PMID: 23145001 Free PMC article.
-
Loss of PSP94 expression is associated with early PSA recurrence and deteriorates outcome of PTEN deleted prostate cancers.Cancer Biol Med. 2019 May;16(2):319-330. doi: 10.20892/j.issn.2095-3941.2018.0384. Cancer Biol Med. 2019. PMID: 31516752 Free PMC article.
-
The rs10993994 in the proximal MSMB promoter region is a functional polymorphism in Asian Indian subjects.Springerplus. 2015 Jul 28;4:380. doi: 10.1186/s40064-015-1164-7. eCollection 2015. Springerplus. 2015. PMID: 26240778 Free PMC article.
-
Growth inhibition properties of the putative prostate cancer biomarkers PSP94 and CRISP-3.Asian J Androl. 2011 Mar;13(2):205-6. doi: 10.1038/aja.2010.120. Epub 2010 Nov 22. Asian J Androl. 2011. PMID: 21102472 Free PMC article. No abstract available.
-
Screening differentially expressed proteins of coronary heart disease with congenital cold syndrome based on tandem mass tag (TMT) technology.Bioengineered. 2021 Dec;12(1):1338-1350. doi: 10.1080/21655979.2021.1912546. Bioengineered. 2021. PMID: 33904367 Free PMC article.
References
-
- Lilja H, Abrahamsson PA. Three predominant proteins secreted by the human prostate gland. Prostate. 1988;12:29–38. - PubMed
-
- Shukeir N, Garde S, Wu JJ, Panchal C, Rabbani SA. Prostate secretory protein of 94 amino acids (PSP-94) and its peptide (PCK3145) as potential therapeutic modalities for prostate cancer. Anti-Cancer Drugs. 2005;16:1045–51. - PubMed
-
- Garde SV, Basrur VS, Li L, Finkelman MA, Krishan A, et al. Prostate secretory protein (PSP94) suppresses the growth of androgen-independent prostate cancer cell line (PC3) and xenografts by inducing apoptosis. Prostate. 1999;38:118–25. - PubMed
-
- Cadieux PA, Mikolajczak SA, Reeves J, Strathdee C, Reid G, et al. Rat PSP94 inhibits the growth and viability of the rat adenocarcinoma cell line PAIII in vitro. Cancer Invest. 2006;24:242–51. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

