p66(Shc) restrains Ras hyperactivation and suppresses metastatic behavior
- PMID: 20676142
- PMCID: PMC3045677
- DOI: 10.1038/onc.2010.326
p66(Shc) restrains Ras hyperactivation and suppresses metastatic behavior
Abstract
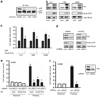
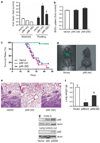
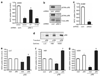
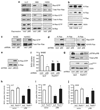
Normal tissue cells survive and proliferate only while anchored to solid substrate. Conversely, transformed cells both survive and proliferate following detachment, having lost attachment context through unclear mechanisms. p66(Shc) is a focal adhesion-associated protein that reports cell attachment through a RhoA-dependent mechanosensory test. We find that human small cell lung cancer (SCLC) cells and mouse Lewis lung carcinoma (LLC), which display aggressive metastatic behavior, lack both p66(Shc) and retinoblastoma (pRB) and bypass anoikis. Re-expression of p66(Shc) in these cells restores anoikis and provides striking protection from metastasis by LLC cells in vivo. Notably, knockdown of p66(Shc) in normal epithelial cells leads to unrestrained Ras activation, preventing anoikis through downstream suppression of RhoA but blocking proliferation in a pRB-dependent manner, thus mimicking oncogenic Ras. Conversely, LLC and SCLC cells display constitutive Ras activation necessary to bypass anoikis, which is reversed by re-expression of p66(Shc). p66(Shc) therefore coordinates Ras-dependent control of proliferation and anchorage sensation, which can be defeated in the evolution of highly metastatic tumors by combined loss of both p66(Shc) and pRB.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Comment in
-
p66(Shc) and Ras: controlling anoikis from the inside-out.Oncogene. 2010 Oct 14;29(41):5556-8. doi: 10.1038/onc.2010.347. Epub 2010 Aug 16. Oncogene. 2010. PMID: 20711240
References
-
- Attwell S, Roskelley C, Dedhar S. The integrin-linked kinase (ILK) suppresses anoikis. Oncogene. 2000;19:3811–3815. - PubMed
-
- Douma S, Van Laar T, Zevenhoven J, Meuwissen R, Van Garderen E, Peeper DS. Suppression of anoikis and induction of metastasis by the neurotrophic receptor TrkB. Nature. 2004;430:1034–1039. - PubMed
-
- Giorgio M, Migliaccio E, Orsini F, Paolucci D, Moroni M, Contursi C, et al. Electron transfer between cytochrome c and p66Shc generates reactive oxygen species that trigger mitochondrial apoptosis. Cell. 2005;122:221–233. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

