Multiphase flow models of biogels from crawling cells to bacterial biofilms
- PMID: 20676304
- PMCID: PMC2880026
- DOI: 10.2976/1.3291142
Multiphase flow models of biogels from crawling cells to bacterial biofilms
Abstract
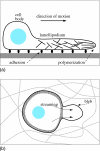
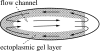
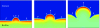
This article reviews multiphase descriptions of the fluid mechanics of cytoplasm in crawling cells and growing bacterial biofilms. These two systems involve gels, which are mixtures composed of a polymer network permeated by water. The fluid mechanics of these systems is essential to their biological function and structure. Their mathematical descriptions must account for the mechanics of the polymer, the water, and the interaction between these two phases. This review focuses on multiphase flow models because this framework is natural for including the relative motion between the phases, the exchange of material between phases, and the additional stresses within the network that arise from nonspecific chemical interactions and the action of molecular motors. These models have been successful in accounting for how different forces are generated and transmitted to achieve cell motion and biofilm growth and they have demonstrated how emergent structures develop though the interactions of the two phases. A short description of multiphase flow models of tumor growth is included to highlight the flexibility of the model in describing diverse biological applications.
Figures

Similar articles
-
Multicomponent hydrodynamic model for heterogeneous biofilms: two-dimensional numerical simulations of growth and interaction with flows.Phys Rev E Stat Nonlin Soft Matter Phys. 2012 Mar;85(3 Pt 1):031908. doi: 10.1103/PhysRevE.85.031908. Epub 2012 Mar 9. Phys Rev E Stat Nonlin Soft Matter Phys. 2012. PMID: 22587124
-
Hybrid Model of Bacterial Biofilm Growth.Bull Math Biol. 2020 Feb 1;82(2):27. doi: 10.1007/s11538-020-00701-6. Bull Math Biol. 2020. PMID: 32008118
-
A multiphase theory for spreading microbial swarms and films.Elife. 2019 Apr 30;8:e42697. doi: 10.7554/eLife.42697. Elife. 2019. PMID: 31038122 Free PMC article.
-
Mini-review: convection around biofilms.Biofouling. 2012;28(2):187-98. doi: 10.1080/08927014.2012.662641. Biofouling. 2012. PMID: 22352315 Review.
-
Physico-chemistry from initial bacterial adhesion to surface-programmed biofilm growth.Adv Colloid Interface Sci. 2018 Nov;261:1-14. doi: 10.1016/j.cis.2018.10.005. Epub 2018 Oct 24. Adv Colloid Interface Sci. 2018. PMID: 30376953 Review.
Cited by
-
A high-resolution finite-difference method for simulating two-fluid, viscoelastic gel dynamics.J Nonnewton Fluid Mech. 2011 Oct;166(19-20):1137-1157. doi: 10.1016/j.jnnfm.2011.07.002. Epub 2011 Jul 18. J Nonnewton Fluid Mech. 2011. PMID: 40771873 Free PMC article.
-
A comparison of computational models for eukaryotic cell shape and motility.PLoS Comput Biol. 2012;8(12):e1002793. doi: 10.1371/journal.pcbi.1002793. Epub 2012 Dec 27. PLoS Comput Biol. 2012. PMID: 23300403 Free PMC article. Review.
-
On a poroviscoelastic model for cell crawling.J Math Biol. 2015 Jan;70(1-2):133-71. doi: 10.1007/s00285-014-0755-1. Epub 2014 Feb 8. J Math Biol. 2015. PMID: 24509816
-
Active random forces can drive differential cellular positioning and enhance motor-driven transport.Mol Biol Cell. 2020 Sep 15;31(20):2283-2288. doi: 10.1091/mbc.E19-11-0629. Epub 2020 Jul 29. Mol Biol Cell. 2020. PMID: 32726176 Free PMC article.
-
Transition from Actin-Driven to Water-Driven Cell Migration Depends on External Hydraulic Resistance.Biophys J. 2018 Jun 19;114(12):2965-2973. doi: 10.1016/j.bpj.2018.04.045. Biophys J. 2018. PMID: 29925032 Free PMC article.
References
-
- Alt, W (2003). “Nonlinear hyperbolic systems of generalized Navier–Stokes type for interactive motion in biology.” In Geometric Analysis and Nonlinear Partial Differential Equations, Hildebrandt, S, and Karcher, H (eds.), Springer, Berlin.
LinkOut - more resources
Full Text Sources
