Vaccination with a human papillomavirus (HPV)-16/18 AS04-adjuvanted cervical cancer vaccine in Korean girls aged 10-14 years
- PMID: 20676333
- PMCID: PMC2908791
- DOI: 10.3346/jkms.2010.25.8.1197
Vaccination with a human papillomavirus (HPV)-16/18 AS04-adjuvanted cervical cancer vaccine in Korean girls aged 10-14 years
Erratum in
- J Korean Med Sci. 2010 Dec;25(12):1831. Ng, Timothy [added]; Bi, Dan [added]; OK, Jin-Ju [added]; Descamps, Dominique [added]; Bock, Hans L [added]
Abstract
The human papillomavirus (HPV)-16/18 AS04-adjuvanted cervical cancer vaccine has been demonstrated to be highly efficacious and immunogenic with a favorable safety profile. This study assessed the immunogenicity and safety of the HPV-16/18 AS04-adjuvanted vaccine in healthy Korean girls aged 10-14 yr. This multi-center, observer-blind trial randomly assigned 321 healthy girls to receive three doses (0, 1, 6-month schedule) of HPV-16/18 AS04-adjuvanted vaccine or hepatitis A vaccine. Immunogenicity against vaccine antigens was assessed one month post-Dose 3. Solicited and unsolicited adverse events (AEs) and serious AEs (SAEs) were recorded. In the according-to-protocol analysis, all initially seronegative subjects vaccinated with the HPV-16/18 AS04-adjuvanted vaccine had seroconverted at Month 7, with a peak geometric mean titer (GMT) that was 600-fold higher than the natural infection titer of 29.8 EU/mL for HPV-16 and a peak GMT that was 400-fold higher than the natural infection titer of 22.6 EU/mL for HPV-18. The vaccine was well tolerated with no increase in reactogenicity with subsequent doses and no reports of vaccine-related SAEs. In conclusion, the HPV-16/18 AS04-adjuvanted vaccine is shown to be highly immunogenic and generally well-tolerated in Korean girls aged 10-14 yr.
Keywords: AS04-adjuvanted; Adolescent; HPV-16/18; Human papillomavirus; Immunogenicity; Prophylactic Vaccine; Safety; Uterine Cervical Neoplasms; VLP.
Figures
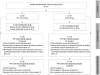
References
-
- Castellsague X, de Sanjose S, Aguado T, Louie KS, Bruni L, Munoz J, Diaz M, Irwin K, Gacic M, Beauvais O, Albero G, Ferrer E, Byrne S, Bosch FX. HPV and Cervical Cancer in the World: 2007 Report. WHO/ICO Information Centre on HPV and Cervical Cancer (HPV Information Centre) Vaccine. 2007;25(Suppl 3):C1–C26. - PubMed
-
- Ministry of Health and Welfare, Korea. 2002 Annual Report of Korea Central Cancer Registry. Seoul, South Korea: Ministry of Health and Welfare; 2003.
-
- Ferlay J, Bray F, Pisani P, Parkin DM. GLOBOCAN 2002. Lyon: IARC Press; 2004. Cancer Incidence, Mortality and Prevalence Worldwide IARC CancerBase No. 5. version 2.0. Available at http://www-dep.iarc.fr/
-
- Shin HR, Park S, Hwang SY, Kim JE, Jung KW, Won YJ, Hwang SS, Yim SH, Choi KS, Park EC, Park SY, Kim JW, Lee HP. Trends in cervical cancer mortality in Korea 1993-2002: Corrected mortality using national death certification data and national cancer incidence data. Int J Cancer. 2008;122:393–397. - PubMed
-
- Chung HH, Jang MJ, Jung KW, Won YJ, Shin HR, Kim JW, Lee HP. Members for Gynecologic Oncology Committee of Korean Society of Obstetrics and Gynecology. Cervical cancer incidence and survival in Korea 1993-2002. Int J Gynecol Cancer. 2006;16:1833–1838. - PubMed

