Two hemocyte lineages exist in silkworm larval hematopoietic organ
- PMID: 20676370
- PMCID: PMC2911379
- DOI: 10.1371/journal.pone.0011816
Two hemocyte lineages exist in silkworm larval hematopoietic organ
Abstract
Background: Insects have multiple hemocyte morphotypes with different functions as do vertebrates, however, their hematopoietic lineages are largely unexplored with the exception of Drosophila melanogaster.
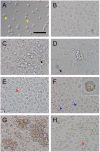
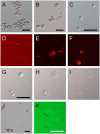
Methodology/principal findings: To study the hematopoietic lineage of the silkworm, Bombyx mori, we investigated in vivo and in vitro differentiation of hemocyte precursors in the hematopoietic organ (HPO) into the four mature hemocyte subsets, namely, plasmatocytes, granulocytes, oenocytoids, and spherulocytes. Five days after implantation of enzymatically-dispersed HPO cells from a GFP-expressing transgenic line into the hemocoel of normal larvae, differentiation into plasmatocytes, granulocytes and oenocytoids, but not spherulocytes, was observed. When the HPO cells were cultured in vitro, plasmatocytes appeared rapidly, and oenocytoids possessing prophenol oxidase activity appeared several days later. HPO cells were also able to differentiate into a small number of granulocytes, but not into spherulocytes. When functionally mature plasmatocytes were cultured in vitro, oenocytoids were observed 10 days later. These results suggest that the hemocyte precursors in HPO first differentiate into plasmatocytes, which further change into oenocytoids.
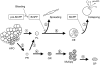
Conclusions/significance: From these results, we propose that B. mori hemocytes can be divided into two major lineages, a granulocyte lineage and a plasmatocyte-oenocytoid lineage. The origins of the spherulocytes could not be determined in this study. We construct a model for the hematopoietic lineages at the larval stage of B. mori.
Conflict of interest statement
Figures


Similar articles
-
Hematopoietic plasticity mapped in Drosophila and other insects.Elife. 2022 Aug 3;11:e78906. doi: 10.7554/eLife.78906. Elife. 2022. PMID: 35920811 Free PMC article. Review.
-
Hemocyte differentiation in the hematopoietic organs of the silkworm, Bombyx mori: prohemocytes have the function of phagocytosis.Cell Tissue Res. 2005 Jun;320(3):535-43. doi: 10.1007/s00441-004-1038-8. Epub 2005 Apr 22. Cell Tissue Res. 2005. PMID: 15846518
-
Silkworm plasmatocytes are more resistant than other hemocyte morphotypes to Bombyx mori nucleopolyhedrovirus infection.J Invertebr Pathol. 2013 Jan;112(1):102-4. doi: 10.1016/j.jip.2012.09.004. Epub 2012 Sep 28. J Invertebr Pathol. 2013. PMID: 23026703
-
Effects of silkworm paralytic peptide on in vitro hematopoiesis and plasmatocyte spreading.Arch Insect Biochem Physiol. 2003 Apr;52(4):163-74. doi: 10.1002/arch.10080. Arch Insect Biochem Physiol. 2003. PMID: 12655604
-
Hemocyte development during Drosophila embryogenesis.Methods Mol Med. 2005;105:109-22. doi: 10.1385/1-59259-826-9:109. Methods Mol Med. 2005. PMID: 15492391 Review.
Cited by
-
Hemocyte Clusters Defined by scRNA-Seq in Bombyx mori: In Silico Analysis of Predicted Marker Genes and Implications for Potential Functional Roles.Front Immunol. 2022 Feb 25;13:852702. doi: 10.3389/fimmu.2022.852702. eCollection 2022. Front Immunol. 2022. PMID: 35281044 Free PMC article.
-
Mechanism of hyperproteinemia-induced blood cell homeostasis imbalance in an animal model.Zool Res. 2022 May 18;43(3):301-318. doi: 10.24272/j.issn.2095-8137.2021.397. Zool Res. 2022. PMID: 35312240 Free PMC article.
-
Hematopoiesis and hematopoietic organs in arthropods.Dev Genes Evol. 2013 Mar;223(1-2):103-15. doi: 10.1007/s00427-012-0428-2. Epub 2013 Jan 15. Dev Genes Evol. 2013. PMID: 23319182 Free PMC article. Review.
-
Hematopoietic plasticity mapped in Drosophila and other insects.Elife. 2022 Aug 3;11:e78906. doi: 10.7554/eLife.78906. Elife. 2022. PMID: 35920811 Free PMC article. Review.
-
Crayfish hemocytes develop along the granular cell lineage.Sci Rep. 2021 Jun 23;11(1):13099. doi: 10.1038/s41598-021-92473-9. Sci Rep. 2021. PMID: 34162929 Free PMC article.
References
-
- Lemaitre B, Hoffmann J. The host defense of Drosophila melanogaster. Annu Rev Immunol. 2007;25:697–743. - PubMed
-
- Wago H. Phagocytic recognition in Bombyx mori. In: Gupta AP, editor. Immunology of Insects and Other Arthropods. Boca Raton: CRC Press; 1991. pp. 215–235.
-
- Lavine MD, Strand MR. Insect hemocytes and their role in immunity. Insect Biochem Mol Biol. 2002;32:1295–1309. - PubMed
-
- Strand MR. The insect cellular immune response. Insect Sci. 2008;15:1–14.
-
- Ashida M, Ochiai M, Niki T. Immunolocalization of prophenoloxidase among hemocytes of the silkworm, Bombyx mori. Tissue Cell. 1988;20:599–610. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

