Does cell lineage in the developing cerebral cortex contribute to its columnar organization?
- PMID: 20676384
- PMCID: PMC2910372
- DOI: 10.3389/fnana.2010.00026
Does cell lineage in the developing cerebral cortex contribute to its columnar organization?
Abstract
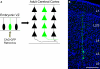
Since the pioneer work of Lorente de Nó, Ramón y Cajal, Brodmann, Mountcastle, Hubel and Wiesel and others, the cerebral cortex has been seen as a jigsaw of anatomic and functional modules involved in the processing of different sets of information. In fact, a columnar distribution of neurons displaying similar functional properties throughout the cerebral cortex has been observed by many researchers. Although it has been suggested that much of the anatomical substrate for such organization would be already specified at early developmental stages, before activity-dependent mechanisms could take place, it is still unclear whether gene expression in the ventricular zone (VZ) could play a role in the development of discrete functional units, such as minicolumns or columns. Cell lineage experiments using replication-incompetent retroviral vectors have shown that the progeny of a single neuroepithelial/radial glial cell in the dorsal telencephalon is organized into discrete radial clusters of sibling excitatory neurons, which have a higher propensity for developing chemical synapses with each other rather than with neighboring non-siblings. Here, we will discuss the possibility that the cell lineage of single neuroepithelial/radial glia cells could contribute for the columnar organization of the neocortex by generating radial columns of sibling, interconnected neurons. Borrowing some concepts from the studies on cell-cell recognition and transcription factor networks, we will also touch upon the potential molecular mechanisms involved in the establishment of sibling-neuron circuits.
Keywords: cell lineage; cortical columns; sister neurons; transcription factors.
Figures

Similar articles
-
Fixing the location and dimensions of functional neocortical columns.HFSP J. 2008 Jun;2(3):132-5. doi: 10.2976/1.2919545. Epub 2008 May 23. HFSP J. 2008. PMID: 19404466 Free PMC article.
-
Specific synapses develop preferentially among sister excitatory neurons in the neocortex.Nature. 2009 Mar 26;458(7237):501-4. doi: 10.1038/nature07722. Epub 2009 Feb 8. Nature. 2009. PMID: 19204731 Free PMC article.
-
Neuronal migration and contact guidance in the primate telencephalon.Postgrad Med J. 1978;54 Suppl 1:25-40. Postgrad Med J. 1978. PMID: 364453 Review.
-
Laminar and columnar auditory cortex in avian brain.Proc Natl Acad Sci U S A. 2010 Jul 13;107(28):12676-81. doi: 10.1073/pnas.1006645107. Epub 2010 Jun 28. Proc Natl Acad Sci U S A. 2010. PMID: 20616034 Free PMC article.
-
The functions of the preplate in development and evolution of the neocortex and hippocampus.Brain Res Brain Res Rev. 1998 Jun;27(1):40-64. doi: 10.1016/s0165-0173(98)00005-8. Brain Res Brain Res Rev. 1998. PMID: 9639671 Review.
Cited by
-
Development of cortical GABAergic innervation.Front Cell Neurosci. 2011 Jul 14;5:14. doi: 10.3389/fncel.2011.00014. eCollection 2011. Front Cell Neurosci. 2011. PMID: 21808605 Free PMC article.
-
Complicated architecture of cortical microcircuit: a comprehensive review.Anat Sci Int. 2025 Aug 20. doi: 10.1007/s12565-025-00877-8. Online ahead of print. Anat Sci Int. 2025. PMID: 40833717 Review.
-
Astrocyte heterogeneity in the brain: from development to disease.Front Cell Neurosci. 2015 Mar 20;9:76. doi: 10.3389/fncel.2015.00076. eCollection 2015. Front Cell Neurosci. 2015. PMID: 25852472 Free PMC article. Review.
-
Hierarchical Modular Structure of the Drosophila Connectome.J Neurosci. 2023 Sep 13;43(37):6384-6400. doi: 10.1523/JNEUROSCI.0134-23.2023. Epub 2023 Aug 17. J Neurosci. 2023. PMID: 37591738 Free PMC article.
-
Alterations in Apical Dendrite Bundling in the Somatosensory Cortex of 5-HT(3A) Receptor Knockout Mice.Front Neuroanat. 2011 Dec 8;5:64. doi: 10.3389/fnana.2011.00064. eCollection 2011. Front Neuroanat. 2011. PMID: 22163214 Free PMC article.
References
-
- Aggoun-Aouaoui D., Kiper D. C., Innocenti G. M. (1996). Growth of callosal terminal arbors in primary visual areas of the cat. Eur. J. Neurosci. 8, 1132–1148 - PubMed
LinkOut - more resources
Full Text Sources

