Characterization of the ebp(fm) pilus-encoding operon of Enterococcus faecium and its role in biofilm formation and virulence in a murine model of urinary tract infection
- PMID: 20676385
- PMCID: PMC2910428
- DOI: 10.4161/viru.1.4.11966
Characterization of the ebp(fm) pilus-encoding operon of Enterococcus faecium and its role in biofilm formation and virulence in a murine model of urinary tract infection
Abstract
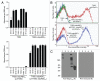
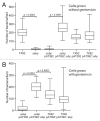
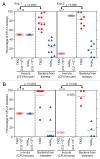
We recently identified 15 genes encoding putative surface proteins with features of MSCRAMMs and/or pili in the Enterococcus faecium TX0016 (DO) genome, including four predicted pilus-encoding gene clusters; we also demonstrated that one of these, ebpABC(fm), is transcribed as an operon, that its putative major pilus subunit, EbpC(fm) (also called pilB), is polymerized into high molecular weight complexes, and that it is enriched among clinical E. faecium isolates. Here, we created a deletion of the ebpABC(fm) operon in an endocarditis-derived E. faecium strain (TX82) and showed, by a combination of whole-cell ELISA, flow cytometry, immunoblot and immunogold electron microscopy, that this deletion abolished EbpC(fm) expression and eliminated EbpC(fm)-containing pili from the cell surface. However, transcription of the downstream sortase, bps(fm), was not affected. Importantly, the ebpABC(fm) deletion resulted in significantly reduced biofilm formation (p < 0.0001) and initial adherence (p < 0.0001) versus the wild-type; both were restored by complementing ebpABC(fm) in trans, which also restored cell surface expression of EbpC(fm) and pilus production. Furthermore, the deletion mutant was significantly attenuated in two independent mixed infection mouse urinary tract experiments, i.e., outnumbered by the wild-type in kidneys (p = 0.0003 and < 0.0001, respectively) and urinary bladders (p = 0.0003 and = 0.002). In conclusion, we have shown that the ebpABC(fm) locus encodes pili on the E. faecium TX82 cell surface and provide the first evidence that pili of this emerging pathogen are important for its ability to form biofilm and to cause infection in an ascending UTI model.
Keywords: Enterococcus faecium; UTI; biofilm; ebp; pathogenesis; pilus; virulence.
Figures



Comment in
-
Insights into the biofilm lifestyle of enterococci.Virulence. 2010 Jul-Aug;1(4):219-21. doi: 10.4161/viru.1.4.12073. Virulence. 2010. PMID: 21178446 No abstract available.
Similar articles
-
Conservation of Ebp-type pilus genes among Enterococci and demonstration of their role in adherence of Enterococcus faecalis to human platelets.Infect Immun. 2011 Jul;79(7):2911-20. doi: 10.1128/IAI.00039-11. Epub 2011 Apr 18. Infect Immun. 2011. PMID: 21502588 Free PMC article.
-
Role of the Emp Pilus Subunits of Enterococcus faecium in Biofilm Formation, Adherence to Host Extracellular Matrix Components, and Experimental Infection.Infect Immun. 2016 Apr 22;84(5):1491-1500. doi: 10.1128/IAI.01396-15. Print 2016 May. Infect Immun. 2016. PMID: 26930703 Free PMC article.
-
The metal ion-dependent adhesion site motif of the Enterococcus faecalis EbpA pilin mediates pilus function in catheter-associated urinary tract infection.mBio. 2012 Jul 24;3(4):e00177-12. doi: 10.1128/mBio.00177-12. Print 2012. mBio. 2012. PMID: 22829678 Free PMC article.
-
Pilus biogenesis of Gram-positive bacteria: Roles of sortases and implications for assembly.Protein Sci. 2017 Aug;26(8):1458-1473. doi: 10.1002/pro.3191. Epub 2017 May 15. Protein Sci. 2017. PMID: 28493331 Free PMC article. Review.
-
Type 1 fimbria and P pili: regulatory mechanisms of the prototypical members of the chaperone-usher fimbrial family.Arch Microbiol. 2024 Aug 10;206(9):373. doi: 10.1007/s00203-024-04092-3. Arch Microbiol. 2024. PMID: 39127787 Free PMC article. Review.
Cited by
-
Contribution of individual Ebp Pilus subunits of Enterococcus faecalis OG1RF to pilus biogenesis, biofilm formation and urinary tract infection.PLoS One. 2013 Jul 11;8(7):e68813. doi: 10.1371/journal.pone.0068813. Print 2013. PLoS One. 2013. PMID: 23874774 Free PMC article.
-
Conservation of Ebp-type pilus genes among Enterococci and demonstration of their role in adherence of Enterococcus faecalis to human platelets.Infect Immun. 2011 Jul;79(7):2911-20. doi: 10.1128/IAI.00039-11. Epub 2011 Apr 18. Infect Immun. 2011. PMID: 21502588 Free PMC article.
-
Structure, Function, and Assembly of Adhesive Organelles by Uropathogenic Bacteria.Microbiol Spectr. 2015 Oct;3(5):10.1128/microbiolspec.UTI-0018-2013. doi: 10.1128/microbiolspec.UTI-0018-2013. Microbiol Spectr. 2015. PMID: 26542038 Free PMC article. Review.
-
Gram-Positive Uropathogens, Polymicrobial Urinary Tract Infection, and the Emerging Microbiota of the Urinary Tract.Microbiol Spectr. 2016 Apr;4(2):10.1128/microbiolspec.UTI-0012-2012. doi: 10.1128/microbiolspec.UTI-0012-2012. Microbiol Spectr. 2016. PMID: 27227294 Free PMC article. Review.
-
Evaluating the Safety of Potential Probiotic Enterococcus durans KLDS6.0930 Using Whole Genome Sequencing and Oral Toxicity Study.Front Microbiol. 2018 Aug 21;9:1943. doi: 10.3389/fmicb.2018.01943. eCollection 2018. Front Microbiol. 2018. PMID: 30186262 Free PMC article.
References
-
- Hidron AI, Edwards JR, Patel J, Horan TC, Sievert DM, Pollock DA, et al. NHSN annual update: antimicrobial-resistant pathogens associated with healthcare-associated infections: annual summary of data reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2006–2007. Infect Control Hosp Epidemiol. 2008;29:996–1011. - PubMed
-
- Hooton TM, Stamm WE. Diagnosis and treatment of uncomplicated urinary tract infection. Infect Dis Clin North Am. 1997;11:551–581. - PubMed
-
- Arias CA, Murray BE. Emergence and management of drug-resistant enterococcal infections. Expert Rev Anti Infect Ther. 2008;6:637–655. - PubMed
-
- Arias CA, Murray BE. Antibiotic-resistant bugs in the 21st century—a clinical super-challenge. N Engl J Med. 2009;360:439–443. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
