The Role of Basic Amino Acids in the Molecular Recognition of Hydroxyapatite by Statherin using Solid State NMR
- PMID: 20676391
- PMCID: PMC2910444
- DOI: 10.1016/j.susc.2010.02.026
The Role of Basic Amino Acids in the Molecular Recognition of Hydroxyapatite by Statherin using Solid State NMR
Abstract
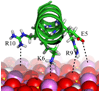
Organisms use proteins such as statherin to control the growth of hydroxyapatite (HAP), which is the principal component of teeth and bone. Though much emphasis has been placed on the acidic character of these proteins, the role of their basic amino acids is not well understood. In this work, solid state nuclear magnetic resonance was used to probe the interaction of the basic arginine side chains with the HAP surface. Statherin samples were individually labeled at each arginine site, and the distance to the surface was measured using the Rotational Echo DOuble Resonance (REDOR) technique. The results indicate a strong coupling between the R9 and R10 residues and the phosphorus atoms on the surface, with internuclear distances of 4.62 ± 0.29 Å and 4.53 ± 0.16 Å, respectively. Conversely, results also indicate weak coupling between R13 and the surface, suggesting this residue is more removed from the surface than R9 and R10. Combining these results with previous data, a new model for the molecular recognition of HAP by statherin is constructed.
Figures



Similar articles
-
Three Decades of REDOR in Protein Science: A Solid-State NMR Technique for Distance Measurement and Spectral Editing.Int J Mol Sci. 2023 Sep 4;24(17):13637. doi: 10.3390/ijms241713637. Int J Mol Sci. 2023. PMID: 37686450 Free PMC article. Review.
-
A (13)C{(31)P} REDOR NMR investigation of the role of glutamic acid residues in statherin- hydroxyapatite recognition.Langmuir. 2009 Oct 20;25(20):12136-43. doi: 10.1021/la901647n. Langmuir. 2009. PMID: 19678690 Free PMC article.
-
Homonuclear and heteronuclear NMR studies of a statherin fragment bound to hydroxyapatite crystals.J Phys Chem B. 2006 May 11;110(18):9324-32. doi: 10.1021/jp056644g. J Phys Chem B. 2006. PMID: 16671751
-
A REDOR NMR study of a phosphorylated statherin fragment bound to hydroxyapatite crystals.J Am Chem Soc. 2005 Jul 6;127(26):9350-1. doi: 10.1021/ja050910m. J Am Chem Soc. 2005. PMID: 15984845
-
Distance measurements to quadrupolar nuclei: Evolution of the rotational echo double resonance technique.Magn Reson Chem. 2021 Sep;59(9-10):908-919. doi: 10.1002/mrc.5150. Epub 2021 Apr 22. Magn Reson Chem. 2021. PMID: 33729630 Review.
Cited by
-
Harnessing biomolecules for bioinspired dental biomaterials.J Mater Chem B. 2020 Oct 14;8(38):8713-8747. doi: 10.1039/d0tb01456g. Epub 2020 Aug 4. J Mater Chem B. 2020. PMID: 32747882 Free PMC article. Review.
-
Three Decades of REDOR in Protein Science: A Solid-State NMR Technique for Distance Measurement and Spectral Editing.Int J Mol Sci. 2023 Sep 4;24(17):13637. doi: 10.3390/ijms241713637. Int J Mol Sci. 2023. PMID: 37686450 Free PMC article. Review.
-
Elastin-Like Protein, with Statherin Derived Peptide, Controls Fluorapatite Formation and Morphology.Front Physiol. 2017 Jun 8;8:368. doi: 10.3389/fphys.2017.00368. eCollection 2017. Front Physiol. 2017. PMID: 28642715 Free PMC article.
-
NMR investigation of the role of osteocalcin and osteopontin at the organic-inorganic interface in bone.Langmuir. 2013 Nov 12;29(45):13873-82. doi: 10.1021/la403203w. Epub 2013 Nov 1. Langmuir. 2013. PMID: 24128197 Free PMC article.
-
Toward a structure determination method for biomineral-associated protein using combined solid- state NMR and computational structure prediction.Structure. 2010 Dec 8;18(12):1678-87. doi: 10.1016/j.str.2010.09.013. Structure. 2010. PMID: 21134646 Free PMC article.
References
-
- Lowenstam H, Weiner S. On Biomineralization. New York: Oxford University Press; 1989.
-
- Simkiss K, Wilbur K. Biomineralization: Cell Biology and Mineral Deposition. New York: Academic Press; 1989.
-
- Weiner S, Addadi L. Acidic macromolecules of mineralized tissues - the controllers of crystal-formation. Trends Biochem. Sci. 1991;16:252–256. - PubMed
-
- Weiner S, Addadi L. Design strategies in mineralized biological materials. J. Mater. Chem. 1997;7:689–702.
-
- Mann S. Molecular tectonics in biomineralization and biomimetic materials chemistry. Nature. 1993;365:499–505.
Grants and funding
LinkOut - more resources
Full Text Sources
