Immunological response to highly active antiretroviral therapy following treatment for prevention of mother to child transmission of HIV-1: a study in Côte d'Ivoire
- PMID: 20678207
- PMCID: PMC2925333
- DOI: 10.1186/1758-2652-13-28
Immunological response to highly active antiretroviral therapy following treatment for prevention of mother to child transmission of HIV-1: a study in Côte d'Ivoire
Abstract
Background: Information is currently limited on the long-term follow up of HIV-1 infected women who are on highly active antiretroviral therapy (HAART) that contains nevirapine and lamivudine and who were previously exposed to antiretroviral drugs for the prevention of mother to child transmission (PMTCT) of HIV.
Methods: We studied the 36-month immunological response to HAART in HIV-1 infected women in Côte d'Ivoire. The women were previously exposed to antiretroviral drug regimens for PMTCT, including single-dose nevirapine and/or short-course zidovudine with or without lamivudine. All HAART regimens included a non-nucleoside reverse transcriptase inhibitor.
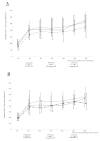
Results: At 36 months: the median absolute increase in CD4+ T cell count was +359 cells/mm3 (IQR: 210-466) in 200 women who had undergone 36-month follow-up visits; +359 cells/mm3 (IQR: 222-491) in 88 women not exposed to PMTCT antiretrovirals; and +363 cells/mm3 (IQR: 200-464) in 112 women exposed to at least one antiretroviral PMTCT regimen. Overall, 49 (19.8%) of the 247 women who initiated HAART met the immunological failure criteria at least once during follow up. The overall probability of immunological failure was 0.08 (95% CI: 0.12-0.15) at 12 months, and 0.21 (95% CI: 0.16-0.27) at 36 months. No difference was observed according to the presence or absence of resistance mutations to nevirapine or lamivudine in women tested at four weeks postpartum. In addition, at 36 months, 23% of women were lost to follow up, dead or had stopped their treatment.
Conclusions: A non-nucleoside reverse transcriptase inhibitor-based antiretroviral regimen, initiated a year or more after PMTCT exposure and that includes nevirapine, remains a good option for at least the first 36 months of treatment.
Figures
Similar articles
-
Maternal 12-month response to antiretroviral therapy following prevention of mother-to-child transmission of HIV type 1, Ivory Coast, 2003-2006.Clin Infect Dis. 2008 Feb 15;46(4):611-21. doi: 10.1086/526780. Clin Infect Dis. 2008. PMID: 18197758
-
Antiretroviral therapy in pregnant women with advanced HIV disease and pregnancy outcomes in Abidjan, Côte d'Ivoire.AIDS. 2008 Sep 12;22(14):1815-20. doi: 10.1097/QAD.0b013e32830b8ab9. AIDS. 2008. PMID: 18753864
-
Antiretroviral treatment and prevention of peripartum and postnatal HIV transmission in West Africa: evaluation of a two-tiered approach.PLoS Med. 2007 Aug;4(8):e257. doi: 10.1371/journal.pmed.0040257. PLoS Med. 2007. PMID: 17713983 Free PMC article.
-
Antiretroviral therapy for prevention of mother-to-child HIV transmission : focus on single-dose nevirapine.Clin Drug Investig. 2006;26(11):611-27. doi: 10.2165/00044011-200626110-00001. Clin Drug Investig. 2006. PMID: 17163296 Review.
-
Current treatment strategies, complications and considerations for the use of HIV antiretroviral therapy during pregnancy.AIDS Rev. 2011 Oct-Dec;13(4):198-213. AIDS Rev. 2011. PMID: 21975356 Review.
Cited by
-
Gender differences in immune reconstitution: a multicentric cohort analysis in sub-Saharan Africa.PLoS One. 2012;7(2):e31078. doi: 10.1371/journal.pone.0031078. Epub 2012 Feb 17. PLoS One. 2012. PMID: 22363550 Free PMC article.
-
Retention of Adult Patients on Antiretroviral Therapy in Low- and Middle-Income Countries: Systematic Review and Meta-analysis 2008-2013.J Acquir Immune Defic Syndr. 2015 May 1;69(1):98-108. doi: 10.1097/QAI.0000000000000553. J Acquir Immune Defic Syndr. 2015. PMID: 25942461 Free PMC article.
-
Long-term clinical and immunologic outcomes of HIV-infected women with and without previous exposure to nevirapine.Trop Med Int Health. 2013 Mar;18(3):344-51. doi: 10.1111/tmi.12054. Epub 2013 Jan 4. Trop Med Int Health. 2013. PMID: 23289497 Free PMC article.
References
-
- Chi BH, Sinkala M, Stringer EM, Cantrell RA, Mtonga V, Bulterys M, Zulu I, Kankasa C, Wilfert C, Weidle PJ, Vermund SH, Stringer JS. Early clinical and immune response to NNRTI-based antiretroviral therapy among women with prior exposure to single-dose nevirapine. AIDS. 2007;13(8):957–964. doi: 10.1097/QAD.0b013e32810996b2. - DOI - PMC - PubMed
-
- Coffie PA, Ekouevi DK, Chaix ML, Tonwe-Gold B, Clarisse AB, Becquet R, Viho I, N'dri-Yoman T, Leroy V, Abrams EJ, Rouzioux C, Dabis F. Maternal 12-month response to antiretroviral therapy following prevention of mother-to-child transmission of HIV type 1, Ivory Coast, 2003-2006. Clin Infect Dis. 2008;13(4):611–621. doi: 10.1086/526780. - DOI - PubMed
-
- Jourdain G, Ngo-Giang-Huong N, Le Coeur S, Bowonwatanuwong C, Kantipong P, Leechanachai P, Ariyadej S, Leenasirimakul P, Hammer S, Lallemant M. Perinatal HIV Prevention Trial Group. Intrapartum exposure to nevirapine and subsequent maternal responses to nevirapine-based antiretroviral therapy. N Engl J Med. 2004;13(3):229–240. doi: 10.1056/NEJMoa041305. - DOI - PubMed
-
- Lockman S, Shapiro RL, Smeaton LM, Wester C, Thior I, Stevens L, Chand F, Makhema J, Moffat C, Asmelash A, Ndase P, Arimi P, van Widenfelt E, Mazhani L, Novitsky V, Lagakos S, Essex M. Response to antiretroviral therapy after a single, peripartum dose of nevirapine. N Engl J Med. 2007;13(2):135–147. doi: 10.1056/NEJMoa062876. - DOI - PubMed
-
- World Health Organization. Antiretroviral drugs for treating pregnant women and preventing HIV infection in infants in resource-limited settings: towards universal access. Recommendations for a public health approach. 2006. http://www.who.int/hiv/pub/mtct/arv_guidelines_mtct.pdf (accessed 26 March 2010)
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

