UHRF1 is a genome caretaker that facilitates the DNA damage response to gamma-irradiation
- PMID: 20678257
- PMCID: PMC2914011
- DOI: 10.1186/2041-9414-1-7
UHRF1 is a genome caretaker that facilitates the DNA damage response to gamma-irradiation
Abstract
Background: DNA double-strand breaks (DSBs) caused by ionizing radiation or by the stalling of DNA replication forks are among the most deleterious forms of DNA damage. The ability of cells to recognize and repair DSBs requires post-translational modifications to histones and other proteins that facilitate access to lesions in compacted chromatin, however our understanding of these processes remains incomplete. UHRF1 is an E3 ubiquitin ligase that has previously been linked to events that regulate chromatin remodeling and epigenetic maintenance. Previous studies have demonstrated that loss of UHRF1 increases the sensitivity of cells to DNA damage however the role of UHRF1 in this response is unclear.
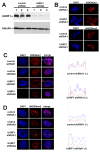
Results: We demonstrate that UHRF1 plays a critical role for facilitating the response to DSB damage caused by gamma-irradiation. UHRF1-depleted cells exhibit increased sensitivity to gamma-irradiation, suggesting a compromised cellular response to DSBs. UHRF1-depleted cells show impaired cell cycle arrest and an impaired accumulation of histone H2AX phosphorylation (gammaH2AX) in response to gamma-irradiation compared to control cells. We also demonstrate that UHRF1 is required for genome integrity, in that UHRF1-depleted cells displayed an increased frequency of chromosomal aberrations compared to control cells.
Conclusions: Our findings indicate a critical role for UHRF1 in maintenance of chromosome integrity and an optimal response to DSB damage.
Figures




Similar articles
-
A cell cycle-dependent BRCA1-UHRF1 cascade regulates DNA double-strand break repair pathway choice.Nat Commun. 2016 Jan 5;7:10201. doi: 10.1038/ncomms10201. Nat Commun. 2016. PMID: 26727879 Free PMC article.
-
Chromatin dynamics and the repair of DNA double strand breaks.Cell Cycle. 2011 Jan 15;10(2):261-7. doi: 10.4161/cc.10.2.14543. Epub 2011 Jan 15. Cell Cycle. 2011. PMID: 21212734 Free PMC article. Review.
-
Chromatin modification and NBS1: their relationship in DNA double-strand break repair.Genes Genet Syst. 2016;90(4):195-208. doi: 10.1266/ggs.15-00010. Epub 2015 Nov 25. Genes Genet Syst. 2016. PMID: 26616756 Review.
-
Chromatin structure and its chemical modifications regulate the ubiquitin ligase substrate selectivity of UHRF1.Proc Natl Acad Sci U S A. 2018 Aug 28;115(35):8775-8780. doi: 10.1073/pnas.1806373115. Epub 2018 Aug 13. Proc Natl Acad Sci U S A. 2018. PMID: 30104358 Free PMC article.
-
Evaluation of the spatial distribution of gammaH2AX following ionizing radiation.J Vis Exp. 2010 Aug 7;(42):2203. doi: 10.3791/2203. J Vis Exp. 2010. PMID: 20736911 Free PMC article.
Cited by
-
Radiation-induced changes in DNA methylation of repetitive elements in the mouse heart.Mutat Res. 2016 May;787:43-53. doi: 10.1016/j.mrfmmm.2016.02.009. Epub 2016 Mar 2. Mutat Res. 2016. PMID: 26963372 Free PMC article.
-
Limoniastrum guyonianum aqueous gall extract induces apoptosis in human cervical cancer cells involving p16 INK4A re-expression related to UHRF1 and DNMT1 down-regulation.J Exp Clin Cancer Res. 2013 May 20;32(1):30. doi: 10.1186/1756-9966-32-30. J Exp Clin Cancer Res. 2013. PMID: 23688286 Free PMC article.
-
Methylation of UHRF1 by SET7 is essential for DNA double-strand break repair.Nucleic Acids Res. 2019 Jan 10;47(1):184-196. doi: 10.1093/nar/gky975. Nucleic Acids Res. 2019. PMID: 30357346 Free PMC article.
-
Comprehensive analysis of single-cell and bulk RNA sequencing reveals postoperative progression markers for non-muscle invasive bladder cancer and predicts responses to immunotherapy.Discov Oncol. 2024 Nov 13;15(1):649. doi: 10.1007/s12672-024-01548-2. Discov Oncol. 2024. PMID: 39532830 Free PMC article.
-
The multi-functionality of UHRF1: epigenome maintenance and preservation of genome integrity.Nucleic Acids Res. 2021 Jun 21;49(11):6053-6068. doi: 10.1093/nar/gkab293. Nucleic Acids Res. 2021. PMID: 33939809 Free PMC article. Review.
References
-
- Muto M, Utsuyama M, Horiguchi T, Kubo E, Sado T, Hirokawa K. The characterization of the monoclonal antibody Th-10a, specific for a nuclear protein appearing in the S phase of the cell cycle in normal thymocytes and its upregulated expression in lymphoma cell lines. Cell Proliferation. 1995;28:645–657. doi: 10.1111/j.1365-2184.1995.tb00051.x. - DOI - PubMed
-
- Arima Y, Hirota T, Bronner C, Mousli M, Fujiwara T, Niwa S-i, Ishikawa H, Saya H. Down-regulation of nuclear protein ICBP90 by p53/p21-dependent DNA-damage checkpoint signals contributes to cell cycle arrest at G1/S transition. Genes to Cells. 2004;9:131–142. doi: 10.1111/j.1356-9597.2004.00710.x. - DOI - PubMed
LinkOut - more resources
Full Text Sources
