Left-right patterning in the mouse requires Epb4.1l5-dependent morphogenesis of the node and midline
- PMID: 20678497
- PMCID: PMC3141284
- DOI: 10.1016/j.ydbio.2010.07.029
Left-right patterning in the mouse requires Epb4.1l5-dependent morphogenesis of the node and midline
Abstract
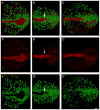
The mouse node is a transient early embryonic structure that is required for left-right asymmetry and for generation of the axial midline, which patterns neural and mesodermal tissues. The node is a shallow teardrop-shaped pit that sits at the distal tip of the early headfold (e7.75) embryo. The shape of the node is believed to be important for generation of the coherent leftward fluid flow required for initiation of left-right asymmetry, but little is known about the morphogenesis of the node. Here we show that the FERM domain protein Lulu/Epb4.1l5 is required for left-right asymmetry in the early mouse embryo. Unlike other genes previously shown to be required for left-right asymmetry in the mouse, lulu is not required for specification of node cell identity, for Nodal signaling in the node or for ciliogenesis. Instead, lulu is required for proper morphogenesis of the node and midline. The precursors of the wild-type node undergo a series of rapid morphological transitions. First, node precursors arise from an epithelial-to-mesenchymal transition at the anterior primitive streak. While in the mesenchymal layer, the node precursors form several ciliated rosette-like clusters; they then rapidly undergo a mesenchymal-to-epithelial transition to insert into the outer, endodermal layer of the embryo. In lulu mutants, node precursor cells are specified and form clusters, but those clusters fail to coalesce to make a single continuous node epithelium. The data suggest that the assembly of the contiguous node epithelium from mesenchymal clusters requires a rapid reorganization of apical-basal polarity that depends on Lulu/Epb4.1l5.
Copyright © 2010 Elsevier Inc. All rights reserved.
Figures







Similar articles
-
The FERM protein Epb4.1l5 is required for organization of the neural plate and for the epithelial-mesenchymal transition at the primitive streak of the mouse embryo.Development. 2007 Jun;134(11):2007-16. doi: 10.1242/dev.000885. Development. 2007. PMID: 17507402
-
Morphogenesis of the node and notochord: the cellular basis for the establishment and maintenance of left-right asymmetry in the mouse.Dev Dyn. 2008 Dec;237(12):3464-76. doi: 10.1002/dvdy.21598. Dev Dyn. 2008. PMID: 18629866 Free PMC article. Review.
-
Tissue-specific requirements for specific domains in the FERM protein Moe/Epb4.1l5 during early zebrafish development.BMC Dev Biol. 2008 Jan 11;8:3. doi: 10.1186/1471-213X-8-3. BMC Dev Biol. 2008. PMID: 18190700 Free PMC article.
-
Cofilin and Vangl2 cooperate in the initiation of planar cell polarity in the mouse embryo.Development. 2013 Mar;140(6):1262-71. doi: 10.1242/dev.085316. Epub 2013 Feb 13. Development. 2013. PMID: 23406901 Free PMC article.
-
Formation and malformation of the vertebrate left-right axis.Curr Mol Med. 2002 Feb;2(1):39-66. doi: 10.2174/1566524023363031. Curr Mol Med. 2002. PMID: 11898848 Review.
Cited by
-
Genetic and biochemical evidence that gastrulation defects in Pofut2 mutants result from defects in ADAMTS9 secretion.Dev Biol. 2016 Aug 1;416(1):111-122. doi: 10.1016/j.ydbio.2016.05.038. Epub 2016 Jun 10. Dev Biol. 2016. PMID: 27297885 Free PMC article.
-
Multicellular rosettes link mesenchymal-epithelial transition to radial intercalation in the mouse axial mesoderm.Dev Cell. 2023 Jun 5;58(11):933-950.e5. doi: 10.1016/j.devcel.2023.03.018. Epub 2023 Apr 19. Dev Cell. 2023. PMID: 37080203 Free PMC article.
-
Cilia in vertebrate left-right patterning.Philos Trans R Soc Lond B Biol Sci. 2016 Dec 19;371(1710):20150410. doi: 10.1098/rstb.2015.0410. Philos Trans R Soc Lond B Biol Sci. 2016. PMID: 27821522 Free PMC article. Review.
-
Rac1 mediates morphogenetic responses to intercellular signals in the gastrulating mouse embryo.Development. 2011 Jul;138(14):3011-20. doi: 10.1242/dev.059766. Development. 2011. PMID: 21693517 Free PMC article.
-
Oct4:Sox2 binding is essential for establishing but not maintaining active and silent states of dynamically regulated genes in pluripotent cells.Genes Dev. 2022 Oct 1;36(19-20):1079-1095. doi: 10.1101/gad.350113.122. Epub 2022 Nov 23. Genes Dev. 2022. PMID: 36418052 Free PMC article.
References
-
- Amack JD, Wang X, Yost HJ. Two T-box genes play independent and cooperative roles to regulate morphogenesis of ciliated Kupffer’s vesicle in zebrafish. Dev Biol. 2007;310:196–210. - PubMed
-
- Ang SL, Rossant J. HNF–3β is essential for node and notochord formation in mouse development. Cell. 1994;78:561–74. - PubMed
-
- Basu B, Brueckner M. Cilia multifunctional organelles at the center of vertebrate left-right asymmetry. Curr Top Dev Biol. 2008;85:151–74. - PubMed
-
- Beddington SP. An autoradiographic analysis of the potency of embryonic ectoderm in the 8th day postimplantation mouse embryo. J Embryol Exp Morphol. 1981;64:87–104. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

