Steroid receptor coactivator-2 expression in brain and physical associations with steroid receptors
- PMID: 20678994
- PMCID: PMC2921768
- DOI: 10.1016/j.neuroscience.2010.05.053
Steroid receptor coactivator-2 expression in brain and physical associations with steroid receptors
Abstract
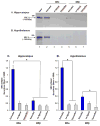
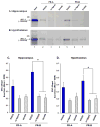
Estradiol and progesterone bind to their respective receptors in the hypothalamus and hippocampus to influence a variety of behavioral and physiological functions, including reproduction and cognition. Work from our lab and others has shown that the nuclear receptor coactivators, steroid receptor coactivator-1 (SRC-1) and SRC-2, are essential for efficient estrogen receptor (ER) and progestin receptor (PR) transcriptional activity in brain and for hormone-dependent behaviors. While the expression of SRC-1 in brain has been studied extensively, little is known about the expression of SRC-2 in brain. In the present studies, we found that SRC-2 was highly expressed throughout the hippocampus, amygdala and hypothalamus, including the medial preoptic area (MPOA), ventral medial nucleus (VMN), arcuate nucleus (ARC), bed nucleus of the stria terminalis, supraoptic nucleus and suprachiasmatic nucleus. In order for coactivators to function with steroid receptors, they must be expressed in the same cells. Indeed, SRC-2 and ER(alpha) were coexpressed in many cells in the MPOA, VMN and ARC, all brain regions known to be involved in female reproductive behavior and physiology. While in vitro studies indicate that SRC-2 physically associates with ER and PR, very little is known about receptor-coactivator interactions in brain. Therefore, we used pull-down assays to test the hypotheses that SRC-2 from hypothalamic and hippocampal tissue physically associate with ER and PR subtypes in a ligand-dependent manner. SRC-2 from both brain regions interacted with ER(alpha) bound to agonist, but not in the absence of ligand or in the presence of the selective ER modulator, tamoxifen. Analysis by mass spectrometry confirmed these ligand-dependent interactions between ER(alpha) and SRC-2 from brain. In dramatic contrast, SRC-2 from brain showed little to no interaction with ERbeta. Interestingly, SRC-2 from both brain regions interacted with PR-B, but not PR-A, in a ligand-dependent manner. Taken together, these findings reveal that SRC-2 is expressed in brain regions known to mediate a variety of steroid-dependent functions. Furthermore, SRC-2 is expressed in many ER(alpha) containing cells in the hypothalamus. Finally, SRC-2 from brain interacts with ER and PR in a subtype-specific manner, which may contribute to the functional differences of these steroid receptor subtypes in brain.
Copyright (c) 2010 IBRO. Published by Elsevier Ltd. All rights reserved.
Figures


Similar articles
-
Steroid receptor coactivator-1 from brain physically interacts differentially with steroid receptor subtypes.Endocrinology. 2008 Oct;149(10):5272-9. doi: 10.1210/en.2008-0048. Epub 2008 Jun 19. Endocrinology. 2008. PMID: 18566116 Free PMC article.
-
Cells in behaviourally relevant brain regions coexpress nuclear receptor coactivators and ovarian steroid receptors.J Neuroendocrinol. 2007 Apr;19(4):262-71. doi: 10.1111/j.1365-2826.2007.01526.x. J Neuroendocrinol. 2007. PMID: 17244199 Free PMC article.
-
Nuclear receptor coactivators are coexpressed with steroid receptors and regulated by estradiol in mouse brain.Neuroendocrinology. 2011;94(1):49-57. doi: 10.1159/000323780. Epub 2011 Feb 9. Neuroendocrinology. 2011. PMID: 21311177 Free PMC article.
-
Physiological consequences of membrane-initiated estrogen signaling in the brain.Front Biosci (Landmark Ed). 2011 Jan 1;16(4):1560-73. doi: 10.2741/3805. Front Biosci (Landmark Ed). 2011. PMID: 21196248 Free PMC article. Review.
-
Cyclin dependent kinase 2 and the regulation of human progesterone receptor activity.Steroids. 2007 Feb;72(2):202-9. doi: 10.1016/j.steroids.2006.11.025. Epub 2007 Jan 4. Steroids. 2007. PMID: 17207508 Free PMC article. Review.
Cited by
-
Nuclear receptor coactivators: regulators of steroid action in brain and behaviour.J Neuroendocrinol. 2013 Nov;25(11):1209-18. doi: 10.1111/jne.12065. J Neuroendocrinol. 2013. PMID: 23795583 Free PMC article. Review.
-
Steroid Receptor Coactivator 3 Regulates Synaptic Plasticity and Hippocampus-dependent Memory.Neurosci Bull. 2021 Dec;37(12):1645-1657. doi: 10.1007/s12264-021-00741-5. Epub 2021 Jul 6. Neurosci Bull. 2021. PMID: 34228315 Free PMC article.
-
Convergence of multiple mechanisms of steroid hormone action.Horm Metab Res. 2012 Jul;44(8):569-76. doi: 10.1055/s-0032-1306343. Epub 2012 Mar 27. Horm Metab Res. 2012. PMID: 22454239 Free PMC article. Review.
-
Estradiol Preferentially Induces Progestin Receptor-A (PR-A) Over PR-B in Cells Expressing Nuclear Receptor Coactivators in the Female Mouse Hypothalamus.eNeuro. 2015 Aug 13;2(4):ENEURO.0012-15.2015. doi: 10.1523/ENEURO.0012-15.2015. eCollection 2015 Jul-Aug. eNeuro. 2015. PMID: 26465008 Free PMC article.
-
Treatment- and population-dependent activity patterns of behavioral and expression QTLs.PLoS One. 2012;7(2):e31805. doi: 10.1371/journal.pone.0031805. Epub 2012 Feb 16. PLoS One. 2012. PMID: 22359631 Free PMC article.
References
-
- Anzick SL, Kononen J, Walker RL, Azorsa DO, Tanner MM, Guan XY, Sauter G, Kallioniemi OP, Trent JM, Meltzer PS. AIB1, a steroid receptor coactivator amplified in breast and ovarian cancer. Science. 1997;277:965–968. - PubMed
-
- Apostolakis EM, Ramamurphy M, Zhou D, Onate S, O’Malley B. Acute disruption of select steroid receptor coactivators prevents reproductive behavior in rats and unmasks genetic adaptation in knockout mice. Molecular Endocrinology. 2002;16:1511–1523. - PubMed
-
- Bai Y, Giguere V. Isoform-Selective interactions between estrogen receptors and steroid receptor coactivators promoted by estradiol and ErbB-2 signaling in living cells. Molecular Endocrinology. 2003;17:589–599. - PubMed
-
- Blaustein JD. Cytoplasmic estrogen receptors in rat brain: Immunocytochemical evidence using three antibodies with distinct epitopes. Endocrinology. 1992;131:1336–1342. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

