eIF4E phosphorylation promotes tumorigenesis and is associated with prostate cancer progression
- PMID: 20679199
- PMCID: PMC2922605
- DOI: 10.1073/pnas.1005320107
eIF4E phosphorylation promotes tumorigenesis and is associated with prostate cancer progression
Abstract
Translational regulation plays a critical role in the control of cell growth and proliferation. A key player in translational control is eIF4E, the mRNA 5' cap-binding protein. Aberrant expression of eIF4E promotes tumorigenesis and has been implicated in cancer development and progression. The activity of eIF4E is dysregulated in cancer. Regulation of eIF4E is partly achieved through phosphorylation. However, the physiological significance of eIF4E phosphorylation in mammals is not clear. Here, we show that knock-in mice expressing a nonphosphorylatable form of eIF4E are resistant to tumorigenesis in a prostate cancer model. By using a genome-wide analysis of translated mRNAs, we show that the phosphorylation of eIF4E is required for translational up-regulation of several proteins implicated in tumorigenesis. Accordingly, increased phospho-eIF4E levels correlate with disease progression in patients with prostate cancer. Our findings establish eIF4E phosphorylation as a critical event in tumorigenesis. These findings raise the possibility that chemical compounds that prevent the phosphorylation of eIF4E could act as anticancer drugs.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
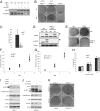
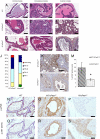
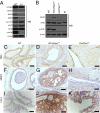

Comment in
-
Mnk earmarks eIF4E for cancer therapy.Proc Natl Acad Sci U S A. 2010 Aug 10;107(32):13975-6. doi: 10.1073/pnas.1008908107. Epub 2010 Aug 2. Proc Natl Acad Sci U S A. 2010. PMID: 20679238 Free PMC article. No abstract available.
-
Re: eIF4E Phosphorylation Promotes Tumorigenesis and is Associated With Prostate Cancer Progression.J Urol. 2011 Apr;185(4):1533. doi: 10.1016/S0022-5347(11)60278-4. J Urol. 2011. PMID: 22098991 No abstract available.
References
-
- Mamane Y, Petroulakis E, LeBacquer O, Sonenberg N. mTOR, translation initiation and cancer. Oncogene. 2006;25:6416–6422. - PubMed
-
- De Benedetti A, Graff JR. eIF-4E expression and its role in malignancies and metastases. Oncogene. 2004;23:3189–3199. - PubMed
-
- Clemens MJ. Targets and mechanisms for the regulation of translation in malignant transformation. Oncogene. 2004;23:3180–3188. - PubMed
-
- Schneider RJ, Sonenberg N. Translational control in cancer development. In: Mathews MB, Sonenberg N, Hershey JWB, editors. Control in Biology and Medicine. Cold Spring Harbor, NY: Cold Spring Harbor Lab Press; 2007. pp. 401–432.
-
- Lazaris-Karatzas A, Montine KS, Sonenberg N. Malignant transformation by a eukaryotic initiation factor subunit that binds to mRNA 5′ cap. Nature. 1990;345:544–547. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

