Th17 plasticity in human autoimmune arthritis is driven by the inflammatory environment
- PMID: 20679229
- PMCID: PMC2930428
- DOI: 10.1073/pnas.1003852107
Th17 plasticity in human autoimmune arthritis is driven by the inflammatory environment
Abstract
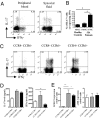
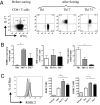
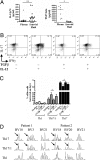
In several murine models of autoimmune arthritis, Th17 cells are the dominant initiators of inflammation. In human arthritis the majority of IL-17-secreting cells within the joint express a cytokine phenotype intermediate between Th17 and Th1. Here we show that Th17/1 cells from the joints of children with inflammatory arthritis express high levels of both Th17 and Th1 lineage-specific transcription factors, RORC2 and T-bet. Modeling the generation of Th17/1 in vitro, we show that Th17 cells "convert" to Th17/1 under conditions that mimic the disease site, namely low TGFbeta and high IL-12 levels, whereas Th1 cells cannot convert to Th17. Th17/1 cells from the inflamed joint share T-cell receptor (TCR) clonality with Th17 cells, suggesting a shared clonal origin between Th17 and Th17/1 cells in arthritis. Using CD161, a lectin-like receptor that is a marker of human Th17, we show synovial Th17 and Th17/1 cells, and unexpectedly, a large proportion of Th1 cells express CD161. We provide evidence to support a Th17 origin for Th1 cells expressing CD161. In vitro, Th17 cells that convert to a Th1 phenotype maintain CD161 expression. In the joint CD161+ Th1 cells share features with Th17 cells, with shared TCR clonality, expression of RORC2 and CCR6 and response to IL-23, although they are IL-17 negative. We propose that the Th17 phenotype may be unstable and that Th17 cells may convert to Th17/1 and Th1 cells in human arthritis. Therefore therapies targeting the induction of Th17 cells could also attenuate Th17/1 and Th1 effector populations within the inflamed joint.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

