Direct inhibition of P/Q-type voltage-gated Ca2+ channels by Gem does not require a direct Gem/Cavbeta interaction
- PMID: 20679232
- PMCID: PMC2930421
- DOI: 10.1073/pnas.1007543107
Direct inhibition of P/Q-type voltage-gated Ca2+ channels by Gem does not require a direct Gem/Cavbeta interaction
Abstract
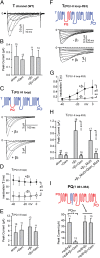
The Rem, Rem2, Rad, and Gem/Kir (RGK) family of small GTP-binding proteins potently inhibits high voltage-activated (HVA) Ca(2+) channels, providing a powerful means of modulating neural, endocrine, and muscle functions. The molecular mechanisms of this inhibition are controversial and remain largely unclear. RGK proteins associate directly with Ca(2+) channel beta subunits (Ca(v)beta), and this interaction is widely thought to be essential for their inhibitory action. In this study, we investigate the molecular underpinnings of Gem inhibition of P/Q-type Ca(2+) channels. We find that a purified Gem protein markedly and acutely suppresses P/Q channel activity in inside-out membrane patches, that this action requires Ca(v)beta but not the Gem/Ca(v)beta interaction, and that Gem coimmunoprecipitates with the P/Q channel alpha(1) subunit (Ca(v)alpha(1)) in a Ca(v)beta-independent manner. By constructing chimeras between P/Q channels and Gem-insensitive low voltage-activated T-type channels, we identify a region encompassing transmembrane segments S1, S2, and S3 in the second homologous repeat of Ca(v)alpha(1) critical for Gem inhibition. Exchanging this region between P/Q and T channel Ca(v)alpha(1) abolishes Gem inhibition of P/Q channels and confers Ca(v)beta-dependent Gem inhibition to a chimeric T channel that also carries the P/Q I-II loop (a cytoplasmic region of Ca(v)alpha(1) that binds Ca(v)beta). Our results challenge the prevailing view regarding the role of Ca(v)beta in RGK inhibition of high voltage-activated Ca(2+) channels and prompt a paradigm in which Gem directly binds and inhibits Ca(v)beta-primed Ca(v)alpha(1) on the plasma membrane.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Catterall WA. Structure and regulation of voltage-gated Ca2+ channels. Annu Rev Cell Dev Biol. 2000;16:521–555. - PubMed
-
- Dolphin AC. β subunits of voltage-gated calcium channels. J Bioenerg Biomembr. 2003;35:599–620. - PubMed
-
- Maltez JM, Nunziato DA, Kim J, Pitt GS. Essential Ca(V)β modulatory properties are AID-independent. Nat Struct Mol Biol. 2005;12:372–377. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

