Polo-like kinase 1 phosphorylation of p150Glued facilitates nuclear envelope breakdown during prophase
- PMID: 20679239
- PMCID: PMC2930408
- DOI: 10.1073/pnas.1006615107
Polo-like kinase 1 phosphorylation of p150Glued facilitates nuclear envelope breakdown during prophase
Abstract
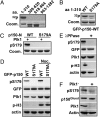
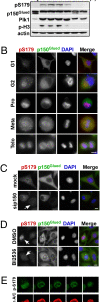
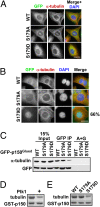
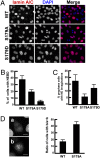
Nuclear envelope breakdown (NEBD) is an essential step during the G2/M transition in higher eukaryotic cells. Increasing evidence supports the notion that both microtubules and microtubule-associated motor proteins are critical regulators of NEBD. Although it has been described that p150(Glued), the major component of the dynein/dynactin complex, localizes in the nuclear envelope during prophase, the exact role of p150(Glued) and its regulation during NEBD are largely elusive. Polo-like kinase 1 (Plk1), the best characterized Ser/Thr kinase, is involved in mitotic entry in several systems; however, the targets of Plk1 during NEBD are unknown. Herein, we show that in mammalian cells both Plk1 and p150(Glued) regulate NEBD and that Plk1 interacts with and phosphoryates p150(Glued) during NEBD at prophase. Using various approaches, we showed that Plk1 phosphorylates p150(Glued) at Ser-179 and that the pS179 epitope is generated at the nuclear envelope of prophase cells. Significantly, Plk1-mediated phosphorylation of p150(Glued) at Ser-179 positively regulates its accumulation at the nuclear envelope during prophase. Finally, we found that cells expressing the Plk1-unphosphorylatable mutant (p150(Glued)-S179A) arrest at G2, as indicated by reduced NEBD, increased levels of cyclin B and phospho-H3, but a decreased level of Cdc2 kinase activity. Taking these data together, we conclude that Plk1 phosphorylation of p150(Glued) might be one major pathway of NEBD regulation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Plk1-dependent microtubule dynamics promotes androgen receptor signaling in prostate cancer.Prostate. 2013 Sep;73(12):1352-63. doi: 10.1002/pros.22683. Epub 2013 May 9. Prostate. 2013. PMID: 23661607 Free PMC article.
-
Polo-like kinase1 is required for recruitment of dynein to kinetochores during mitosis.J Biol Chem. 2011 Jun 10;286(23):20769-77. doi: 10.1074/jbc.M111.226605. Epub 2011 Apr 20. J Biol Chem. 2011. PMID: 21507953 Free PMC article.
-
Aurora A contributes to p150(glued) phosphorylation and function during mitosis.J Cell Biol. 2010 May 17;189(4):651-9. doi: 10.1083/jcb.201001144. J Cell Biol. 2010. PMID: 20479466 Free PMC article.
-
Multiple Roles of PLK1 in Mitosis and Meiosis.Cells. 2023 Jan 2;12(1):187. doi: 10.3390/cells12010187. Cells. 2023. PMID: 36611980 Free PMC article. Review.
-
Microscopy-induced radiation damage, microtubules, and progression through the terminal stage of G2 (prophase) in vertebrate somatic cells.Cold Spring Harb Symp Quant Biol. 2000;65:369-76. doi: 10.1101/sqb.2000.65.369. Cold Spring Harb Symp Quant Biol. 2000. PMID: 12760052 Review. No abstract available.
Cited by
-
Plk1 Inhibitors in Cancer Therapy: From Laboratory to Clinics.Mol Cancer Ther. 2016 Jul;15(7):1427-35. doi: 10.1158/1535-7163.MCT-15-0897. Epub 2016 Jun 21. Mol Cancer Ther. 2016. PMID: 27330107 Free PMC article. Review.
-
Plk1-dependent microtubule dynamics promotes androgen receptor signaling in prostate cancer.Prostate. 2013 Sep;73(12):1352-63. doi: 10.1002/pros.22683. Epub 2013 May 9. Prostate. 2013. PMID: 23661607 Free PMC article.
-
Nde1 and Ndel1: Outstanding Mysteries in Dynein-Mediated Transport.Front Cell Dev Biol. 2022 Apr 12;10:871935. doi: 10.3389/fcell.2022.871935. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 35493069 Free PMC article. Review.
-
Mitotic Disassembly of Nuclear Pore Complexes Involves CDK1- and PLK1-Mediated Phosphorylation of Key Interconnecting Nucleoporins.Dev Cell. 2017 Oct 23;43(2):141-156.e7. doi: 10.1016/j.devcel.2017.08.020. Dev Cell. 2017. PMID: 29065306 Free PMC article.
-
Polo-like kinase 1, on the rise from cell cycle regulation to prostate cancer development.Protein Cell. 2012 Mar;3(3):182-97. doi: 10.1007/s13238-012-2020-y. Epub 2012 Mar 23. Protein Cell. 2012. PMID: 22447658 Free PMC article. Review.
References
-
- Salina D, et al. Cytoplasmic dynein as a facilitator of nuclear envelope breakdown. Cell. 2002;108:97–107. - PubMed
-
- King SJ, Schroer TA. Dynactin increases the processivity of the cytoplasmic dynein motor. Nat Cell Biol. 2000;2:20–24. - PubMed
-
- Karki S, Holzbaur EL. Affinity chromatography demonstrates a direct binding between cytoplasmic dynein and the dynactin complex. J Biol Chem. 1995;270:28806–28811. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

