Intermolecular associations determine the dynamics of the circadian KaiABC oscillator
- PMID: 20679240
- PMCID: PMC2930409
- DOI: 10.1073/pnas.1002119107
Intermolecular associations determine the dynamics of the circadian KaiABC oscillator
Abstract
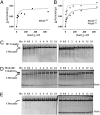
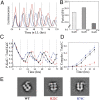
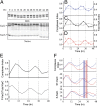
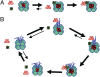
Three proteins from cyanobacteria (KaiA, KaiB, and KaiC) can reconstitute circadian oscillations in vitro. At least three molecular properties oscillate during this reaction, namely rhythmic phosphorylation of KaiC, ATP hydrolytic activity of KaiC, and assembly/disassembly of intermolecular complexes among KaiA, KaiB, and KaiC. We found that the intermolecular associations determine key dynamic properties of this in vitro oscillator. For example, mutations within KaiB that alter the rates of binding of KaiB to KaiC also predictably modulate the period of the oscillator. Moreover, we show that KaiA can bind stably to complexes of KaiB and hyperphosphorylated KaiC. Modeling simulations indicate that the function of this binding of KaiA to the KaiB*KaiC complex is to inactivate KaiA's activity, thereby promoting the dephosphorylation phase of the reaction. Therefore, we report here dynamics of interaction of KaiA and KaiB with KaiC that determine the period and amplitude of this in vitro oscillator.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Circadian oscillations of KaiA-KaiC and KaiB-KaiC complex formations in an in vitro reconstituted KaiABC clock oscillator.Genes Cells. 2016 Aug;21(8):890-900. doi: 10.1111/gtc.12392. Epub 2016 Aug 1. Genes Cells. 2016. PMID: 27477077
-
CryoEM and molecular dynamics of the circadian KaiB-KaiC complex indicates that KaiB monomers interact with KaiC and block ATP binding clefts.J Mol Biol. 2013 Sep 23;425(18):3311-24. doi: 10.1016/j.jmb.2013.06.018. Epub 2013 Jun 22. J Mol Biol. 2013. PMID: 23796516 Free PMC article.
-
Loop-loop interactions regulate KaiA-stimulated KaiC phosphorylation in the cyanobacterial KaiABC circadian clock.Biochemistry. 2013 Feb 19;52(7):1208-20. doi: 10.1021/bi301691a. Epub 2013 Feb 7. Biochemistry. 2013. PMID: 23351065 Free PMC article.
-
A cyanobacterial circadian clock based on the Kai oscillator.Cold Spring Harb Symp Quant Biol. 2007;72:47-55. doi: 10.1101/sqb.2007.72.029. Cold Spring Harb Symp Quant Biol. 2007. PMID: 18419262 Review.
-
The molecular clockwork of a protein-based circadian oscillator.FEBS Lett. 2009 Dec 17;583(24):3938-47. doi: 10.1016/j.febslet.2009.11.021. FEBS Lett. 2009. PMID: 19913541 Free PMC article. Review.
Cited by
-
Discrete gene replication events drive coupling between the cell cycle and circadian clocks.Proc Natl Acad Sci U S A. 2016 Apr 12;113(15):4063-8. doi: 10.1073/pnas.1507291113. Epub 2016 Mar 28. Proc Natl Acad Sci U S A. 2016. PMID: 27035936 Free PMC article.
-
Conversion between two conformational states of KaiC is induced by ATP hydrolysis as a trigger for cyanobacterial circadian oscillation.Sci Rep. 2016 Sep 1;6:32443. doi: 10.1038/srep32443. Sci Rep. 2016. PMID: 27580682 Free PMC article.
-
Metabolic compensation and circadian resilience in prokaryotic cyanobacteria.Annu Rev Biochem. 2014;83:221-47. doi: 10.1146/annurev-biochem-060713-035632. Annu Rev Biochem. 2014. PMID: 24905782 Free PMC article. Review.
-
Dimer dissociation is a key energetic event in the fold-switch pathway of KaiB.Biophys J. 2022 Mar 15;121(6):943-955. doi: 10.1016/j.bpj.2022.02.012. Epub 2022 Feb 11. Biophys J. 2022. PMID: 35151633 Free PMC article.
-
Evolution of KaiC-Dependent Timekeepers: A Proto-circadian Timing Mechanism Confers Adaptive Fitness in the Purple Bacterium Rhodopseudomonas palustris.PLoS Genet. 2016 Mar 16;12(3):e1005922. doi: 10.1371/journal.pgen.1005922. eCollection 2016 Mar. PLoS Genet. 2016. PMID: 26982486 Free PMC article.
References
-
- Ditty JL, Mackey SR, Johnson CH, editors. Bacterial Circadian Programs. Heidelberg: Springer; 2009.
-
- Liu Y, et al. Circadian orchestration of gene expression in cyanobacteria. Genes Dev. 1995;9:1469–1478. - PubMed
-
- Nakajima M, et al. Reconstitution of circadian oscillation of cyanobacterial KaiC phosphorylation in vitro. Science. 2005;308:414–415. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

