Amyloid beta 42 peptide (Abeta42)-lowering compounds directly bind to Abeta and interfere with amyloid precursor protein (APP) transmembrane dimerization
- PMID: 20679249
- PMCID: PMC2930477
- DOI: 10.1073/pnas.1003026107
Amyloid beta 42 peptide (Abeta42)-lowering compounds directly bind to Abeta and interfere with amyloid precursor protein (APP) transmembrane dimerization
Abstract
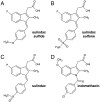
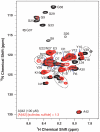
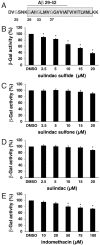
Following ectodomain shedding by beta-secretase, successive proteolytic cleavages within the transmembrane sequence (TMS) of the amyloid precursor protein (APP) catalyzed by gamma-secretase result in the release of amyloid-beta (Abeta) peptides of variable length. Abeta peptides with 42 amino acids appear to be the key pathogenic species in Alzheimer's disease, as they are believed to initiate neuronal degeneration. Sulindac sulfide, which is known as a potent gamma-secretase modulator (GSM), selectively reduces Abeta42 production in favor of shorter Abeta species, such as Abeta38. By studying APP-TMS dimerization we previously showed that an attenuated interaction similarly decreased Abeta42 levels and concomitantly increased Abeta38 levels. However, the precise molecular mechanism by which GSMs modulate Abeta production is still unclear. In this study, using a reporter gene-based dimerization assay, we found that APP-TMS dimers are destabilized by sulindac sulfide and related Abeta42-lowering compounds in a concentration-dependent manner. By surface plasmon resonance analysis and NMR spectroscopy, we show that sulindac sulfide and novel sulindac-derived compounds directly bind to the Abeta sequence. Strikingly, the attenuated APP-TMS interaction by GSMs correlated strongly with Abeta42-lowering activity and binding strength to the Abeta sequence. Molecular docking analyses suggest that certain GSMs bind to the GxxxG dimerization motif in the APP-TMS. We conclude that these GSMs decrease Abeta42 levels by modulating APP-TMS interactions. This effect specifically emphasizes the importance of the dimeric APP-TMS as a promising drug target in Alzheimer's disease.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

