Membrane transport in primitive cells
- PMID: 20679338
- PMCID: PMC2908771
- DOI: 10.1101/cshperspect.a002188
Membrane transport in primitive cells
Abstract
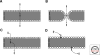
Although model protocellular membranes consisting of monoacyl lipids are similar to membranes composed of contemporary diacyl lipids, they differ in at least one important aspect. Model protocellular membranes allow for the passage of polar solutes and thus can potentially support cell-to functions without the aid of transport machinery. The ability to transport polar molecules likely stems from increased lipid dynamics. Selectively permeable vesicle membranes composed of monoacyl lipids allow for many lifelike processes to emerge from a remarkably small set of molecules.
Figures
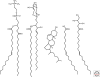
References
-
- Armstrong VT, Brzustowicz MR, Wassall SR, Jenski LJ, Stillwell W 2003. Rapid flip-flop in polyunsaturated (docosahexaenoate) phospholipid membranes. Arch Biochem Biophys 414: 74–82 - PubMed
-
- Benner SA, Hutter D 2002. Phosphates, DNA, and the search for nonterrean life: A second generation model for genetic molecules. Bioorg Chem 30: 62–80 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
