Spectroscopic studies of GSK3{beta} phosphorylation of the neuronal tau protein and its interaction with the N-terminal domain of apolipoprotein E
- PMID: 20679343
- PMCID: PMC2963357
- DOI: 10.1074/jbc.M110.149419
Spectroscopic studies of GSK3{beta} phosphorylation of the neuronal tau protein and its interaction with the N-terminal domain of apolipoprotein E
Abstract
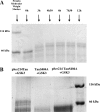
Alzheimer disease neurons are characterized by extraneuronal plaques formed by aggregated amyloid-β peptide and by intraneuronal tangles composed of fibrillar aggregates of the microtubule-associated Tau protein. Tau is mostly found in a hyperphosphorylated form in these tangles. Glycogen synthase kinase 3β (GSK3β) is a proline-directed kinase generally considered as one of the major players that (hyper)phosphorylates Tau. The kinase phosphorylates mainly (Ser/Thr)-Pro motifs and is believed to require a priming activity by another kinase. Here, we use an in vitro phosphorylation assay and NMR spectroscopy to characterize in a qualitative and quantitative manner the phosphorylation of Tau by GSK3β. We find that three residues can be phosphorylated (Ser-396, Ser-400, and Ser-404) by GSK3β alone, without priming. Ser-404 is essential in this process, as its mutation to Ala prevents all activity of GSK3β. However, priming enhances the catalytic efficacy of the kinase, as initial phosphorylation of Ser-214 by the cAMP-dependent protein kinase (PKA) leads to the rapid modification by GSK3β of four regularly spaced additional sites. Because the regular incorporation of negative charges by GSK3β leads to a potential parallel between phospho-Tau and heparin, we investigated its interaction with the heparin/low density lipoprotein receptor binding domain of human apolipoprotein E. We indeed observed an interaction between the GSK3β-promoted regular phospho-pattern on Tau and the apolipoprotein E fragment but none in the absence of phosphorylation or the presence of an irregular phosphorylation pattern by the prolonged activity of PKA. Apolipoprotein E is therefore able to discriminate and interact with specific phosphorylation patterns of Tau.
Figures


References
-
- Buée L., Bussière T., Buée-Scherrer V., Delacourte A., Hof P. R. (2000) Brain Res. Brain Res. Rev. 33, 95–130 - PubMed
-
- Cleveland D. W., Hwo S. Y., Kirschner M. W. (1977) J. Mol. Biol. 116, 227–247 - PubMed
-
- Avila J., Lucas J. J., Perez M., Hernandez F. (2004) Physiol. Rev. 84, 361–384 - PubMed
-
- Braak H., Braak E., Ohm T., Bohl J. (1988) Stain Technol. 63, 197–200 - PubMed
-
- Newell K. L., Hyman B. T., Growdon J. H., Hedley-Whyte E. T. (1999) J. Neuropathol. Exp. Neurol. 58, 1147–1155 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

