The role of Hv1 and CatSper channels in sperm activation
- PMID: 20679352
- PMCID: PMC3010136
- DOI: 10.1113/jphysiol.2010.194142
The role of Hv1 and CatSper channels in sperm activation
Abstract
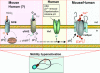
Elevations of sperm intracellular pH and Ca(2+) regulate sperm motility, chemotaxis, capacitation and the acrosome reaction, and play a vital role in the ability of the sperm cell to reach and fertilise the egg. In human spermatozoa, the flagellar voltage-gated proton channel Hv1 is the main H(+) extrusion pathway that controls sperm intracellular pH, and the pH-dependent flagellar Ca²(+) channel CatSper is the main pathway for Ca²(+) entry as measured by the whole-cell patch clamp technique. Hv1 and CatSper channels are co-localized within the principal piece of the sperm flagellum. Hv1 is dedicated to proton extrusion from flagellum and is activated by membrane depolarisation, an alkaline extracellular environment, the endocannabinoid anandamide, and removal of extracellular zinc, a potent Hv1 blocker. The CatSper channel is strongly potentiated by intracellular alkalinisation. Since Hv1 and CatSper channels are located in the same subcellular domain, proton extrusion via Hv1 channels should induce intraflagellar alkalinisation and activate CatSper ion channels. Therefore the combined action of Hv1 and CatSper channels in human spermatozoa can induce elevation of both intracellular pH and Ca²(+) required for sperm activation in the female reproductive tract. Here, we discuss how Hv1 and CatSper channels regulate human sperm physiology and the differences in control of sperm intracellular pH and Ca²(+) between species.
Figures

Similar articles
-
Functional insights into voltage gated proton channel (Hv1) in bull spermatozoa.Theriogenology. 2019 Sep 15;136:118-130. doi: 10.1016/j.theriogenology.2019.06.015. Epub 2019 Jun 17. Theriogenology. 2019. PMID: 31254725
-
Acid extrusion from human spermatozoa is mediated by flagellar voltage-gated proton channel.Cell. 2010 Feb 5;140(3):327-37. doi: 10.1016/j.cell.2009.12.053. Cell. 2010. PMID: 20144758
-
The control of male fertility by spermatozoan ion channels.Annu Rev Physiol. 2012;74:453-75. doi: 10.1146/annurev-physiol-020911-153258. Epub 2011 Oct 13. Annu Rev Physiol. 2012. PMID: 22017176 Free PMC article. Review.
-
Rediscovering sperm ion channels with the patch-clamp technique.Mol Hum Reprod. 2011 Aug;17(8):478-99. doi: 10.1093/molehr/gar044. Epub 2011 Jun 4. Mol Hum Reprod. 2011. PMID: 21642646 Free PMC article. Review.
-
Asymmetrically Positioned Flagellar Control Units Regulate Human Sperm Rotation.Cell Rep. 2018 Sep 4;24(10):2606-2613. doi: 10.1016/j.celrep.2018.08.016. Cell Rep. 2018. PMID: 30184496 Free PMC article.
Cited by
-
Multiple mechanisms contribute to fluorometry signals from the voltage-gated proton channel.Commun Biol. 2022 Oct 26;5(1):1131. doi: 10.1038/s42003-022-04065-6. Commun Biol. 2022. PMID: 36289443 Free PMC article.
-
Molecular Basis of Human Sperm Capacitation.Front Cell Dev Biol. 2018 Jul 27;6:72. doi: 10.3389/fcell.2018.00072. eCollection 2018. Front Cell Dev Biol. 2018. PMID: 30105226 Free PMC article. Review.
-
Zinc is a master-regulator of sperm function associated with binding, motility, and metabolic modulation during porcine sperm capacitation.Commun Biol. 2022 Jun 3;5(1):538. doi: 10.1038/s42003-022-03485-8. Commun Biol. 2022. PMID: 35660793 Free PMC article.
-
Pharmacological Inactivation of CatSper Blocks Sperm Fertilizing Ability Independently of the Capacitation Status of the Cells: Implications for Non-hormonal Contraception.Front Cell Dev Biol. 2021 Jul 6;9:686461. doi: 10.3389/fcell.2021.686461. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34295893 Free PMC article.
-
HV1 acts as a sodium sensor and promotes superoxide production in medullary thick ascending limb of Dahl salt-sensitive rats.Hypertension. 2014 Sep;64(3):541-50. doi: 10.1161/HYPERTENSIONAHA.114.03549. Epub 2014 Jun 16. Hypertension. 2014. PMID: 24935944 Free PMC article.
References
-
- Acott TS, Carr DW. Inhibition of bovine spermatozoa by caudal epididymal fluid: II. Interaction of pH and a quiescence factor. Biol Reprod. 1984;30:926–935. - PubMed
-
- Austin CR. Observations on the penetration of the sperm in the mammalian egg. Aust J Sci Res Ser. 1951;4:581–596. - PubMed
-
- Avidan N, Tamary H, Dgany O, Cattan D, Pariente A, Thulliez M, Borot N, Moati L, Barthelme A, Shalmon L, Krasnov T, Ben-Asher E, Olender T, Khen M, Yaniv I, Zaizov R, Shalev H, Delaunay J, Fellous M, Lancet D, Beckmann JS. CATSPER2, a human autosomal nonsyndromic male infertility gene. Eur J Hum Genet. 2003;11:497–502. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

