IL-2 reverses established type 1 diabetes in NOD mice by a local effect on pancreatic regulatory T cells
- PMID: 20679400
- PMCID: PMC2931175
- DOI: 10.1084/jem.20100209
IL-2 reverses established type 1 diabetes in NOD mice by a local effect on pancreatic regulatory T cells
Abstract
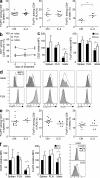
Regulatory T cells (T reg cells) play a major role in controlling the pathogenic autoimmune process in type 1 diabetes (T1D). Interleukin 2 (IL-2), a cytokine which promotes T reg cell survival and function, may thus have therapeutic efficacy in T1D. We show that 5 d of low-dose IL-2 administration starting at the time of T1D onset can reverse established disease in NOD (nonobese diabetic) mice, with long-lasting effects. Low-dose IL-2 increases the number of T reg cells in the pancreas and induces expression of T reg cell-associated proteins including Foxp3, CD25, CTLA-4, ICOS (inducible T cell costimulator), and GITR (glucocorticoid-induced TNF receptor) in these cells. Treatment also suppresses interferon gamma production by pancreas-infiltrating T cells. Transcriptome analyses show that low-dose IL-2 exerts much greater influence on gene expression of T reg cells than effector T cells (T eff cells), suggesting that nonspecific activation of pathogenic T eff cells is less likely. We provide the first preclinical data showing that low-dose IL-2 can reverse established T1D, suggesting that this treatment merits evaluation in patients with T1D.
Figures

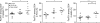


References
-
- Cassan C., Piaggio E., Zappulla J.P., Mars L.T., Couturier N., Bucciarelli F., Desbois S., Bauer J., Gonzalez-Dunia D., Liblau R.S. 2006. Pertussis toxin reduces the number of splenic Foxp3+ regulatory T cells. J. Immunol. 177:1552–1560 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

