Mucosal immunization with Vibrio cholerae outer membrane vesicles provides maternal protection mediated by antilipopolysaccharide antibodies that inhibit bacterial motility
- PMID: 20679439
- PMCID: PMC2950341
- DOI: 10.1128/IAI.00398-10
Mucosal immunization with Vibrio cholerae outer membrane vesicles provides maternal protection mediated by antilipopolysaccharide antibodies that inhibit bacterial motility
Abstract
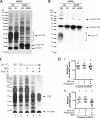
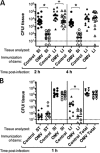
Vibrio cholerae is the causative agent of cholera, a severe diarrheal disease that remains endemic in many parts of the world and can cause outbreaks wherever sanitation and clean water systems break down. Prevention of disease could be achieved through improved sanitation and clean water provision supported by vaccination. V. cholerae serogroup O1 is the major cause of cholera; O1 serotypes Inaba and Ogawa have similar disease burdens, while O139 is the only non-O1 serogroup to cause epidemics. We showed previously that immunization of adult female mice with purified V. cholerae outer membrane vesicles (OMVs) elicits an antibody response that protect neonates from oral V. cholerae challenge and that suckling from an immunized dam accounts for the majority of protection from V. cholerae colonization. Here we report that lipopolysaccharide (LPS) is the major OMV protective antigen. Mucosal immunization with OMVs from Inaba or Ogawa provides significant cross-serotype protection from V. cholerae colonization, although serotype-specific antigens are dominant. OMVs from O1 or O139 do not provide cross-serogroup protection, but by immunization with a mixture of O1 and O139 OMVs, cross-serogroup protection was achieved. Neonatal protection is not associated with significant bacterial death but may involve inhibition of motility, as antibodies from OMV-immunized mice inhibit V. cholerae motility in vitro, with trends that parallel in vivo protection. Motility assays also reveal that a higher antibody titer is required to immobilize O139 compared to O1, a phenotype that is O139 capsule dependent.
Figures




Similar articles
-
Characterization of Vibrio cholerae outer membrane vesicles as a candidate vaccine for cholera.Infect Immun. 2009 Jan;77(1):472-84. doi: 10.1128/IAI.01139-08. Epub 2008 Nov 10. Infect Immun. 2009. PMID: 19001078 Free PMC article.
-
Immunization with Vibrio cholerae outer membrane vesicles induces protective immunity in mice.Infect Immun. 2008 Oct;76(10):4554-63. doi: 10.1128/IAI.00532-08. Epub 2008 Aug 4. Infect Immun. 2008. PMID: 18678672 Free PMC article.
-
Immunogenicity and protective efficacy of Vibrio cholerae outer membrane vesicles in rabbit model.FEMS Immunol Med Microbiol. 2010 Oct;60(1):18-27. doi: 10.1111/j.1574-695X.2010.00692.x. FEMS Immunol Med Microbiol. 2010. PMID: 20528929
-
Epidemiology & molecular biology of Vibrio cholerae O139 Bengal.Indian J Med Res. 1996 Jul;104:14-27. Indian J Med Res. 1996. PMID: 8783504 Review.
-
A review of the current status of enteric vaccines.P N G Med J. 1995 Dec;38(4):325-31. P N G Med J. 1995. PMID: 9522876 Review.
Cited by
-
LPLUNC1 modulates innate immune responses to Vibrio cholerae.J Infect Dis. 2011 Nov;204(9):1349-57. doi: 10.1093/infdis/jir544. Epub 2011 Sep 7. J Infect Dis. 2011. PMID: 21900486 Free PMC article.
-
Vibrio cholerae, classification, pathogenesis, immune response, and trends in vaccine development.Front Med (Lausanne). 2023 May 5;10:1155751. doi: 10.3389/fmed.2023.1155751. eCollection 2023. Front Med (Lausanne). 2023. PMID: 37215733 Free PMC article. Review.
-
Versatile effects of bacterium-released membrane vesicles on mammalian cells and infectious/inflammatory diseases.Acta Pharmacol Sin. 2018 Apr;39(4):514-533. doi: 10.1038/aps.2017.82. Epub 2017 Aug 31. Acta Pharmacol Sin. 2018. PMID: 28858295 Free PMC article. Review.
-
Lipopolysaccharide modifications of a cholera vaccine candidate based on outer membrane vesicles reduce endotoxicity and reveal the major protective antigen.Infect Immun. 2013 Jul;81(7):2379-93. doi: 10.1128/IAI.01382-12. Epub 2013 Apr 29. Infect Immun. 2013. PMID: 23630951 Free PMC article.
-
Impact of Immunoglobulin Isotype and Epitope on the Functional Properties of Vibrio cholerae O-Specific Polysaccharide-Specific Monoclonal Antibodies.mBio. 2021 Apr 20;12(2):e03679-20. doi: 10.1128/mBio.03679-20. mBio. 2021. PMID: 33879588 Free PMC article.
References
-
- Ahmed, T., A. M. Svennerholm, A. Al Tarique, G. N. Sultana, and F. Qadri. 2009. Enhanced immunogenicity of an oral inactivated cholera vaccine in infants in Bangladesh obtained by zinc supplementation and by temporary withholding breast-feeding. Vaccine 27:1433-1439. - PubMed
-
- Alaniz, R. C., B. L. Deatherage, J. C. Lara, and B. T. Cookson. 2007. Membrane vesicles are immunogenic facsimiles of Salmonella typhimurium that potently activate dendritic cells, prime B and T cell responses, and stimulate protective immunity in vivo. J. Immunol. 179:7692-7701. - PubMed
-
- Albert, M. J., K. Alam, M. Ansaruzzaman, F. Qadri, and R. B. Sack. 1994. Lack of cross-protection against diarrhea due to Vibrio cholerae O139 (Bengal strain) after oral immunization of rabbits with V. cholerae O1 vaccine strain CVD103-HgR. J. Infect. Dis. 169:230-231. - PubMed
-
- Albert, M. J., K. Alam, A. S. Rahman, S. Huda, and R. B. Sack. 1994. Lack of cross-protection against diarrhea due to Vibrio cholerae O1 after oral immunization of rabbits with V. cholerae O139 Bengal. J. Infect. Dis. 169:709-710. - PubMed
-
- Albert, M. J., A. K. Siddique, M. S. Islam, A. S. Faruque, M. Ansaruzzaman, S. M. Faruque, and R. B. Sack. 1993. Large outbreak of clinical cholera due to Vibrio cholerae non-O1 in Bangladesh. Lancet 341:704. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

