Three Yersinia pestis adhesins facilitate Yop delivery to eukaryotic cells and contribute to plague virulence
- PMID: 20679446
- PMCID: PMC2950350
- DOI: 10.1128/IAI.00167-10
Three Yersinia pestis adhesins facilitate Yop delivery to eukaryotic cells and contribute to plague virulence
Abstract
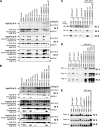
To establish a successful infection, Yersinia pestis requires the delivery of cytotoxic Yops to host cells. Yops inhibit phagocytosis, block cytokine responses, and induce apoptosis of macrophages. The Y. pestis adhesin Ail facilitates Yop translocation and is required for full virulence in mice. To determine the contributions of other adhesins to Yop delivery, we deleted five known adhesins of Y. pestis. In addition to Ail, plasminogen activator (Pla) and pH 6 antigen (Psa) could mediate Yop translocation to host cells. The contribution of each adhesin to binding and Yop delivery was dependent upon the growth conditions. When cells were pregrown at 28°C and pH 7, the order of importance for adhesins in cell binding and cytotoxicity was Ail > Pla > Psa. Y. pestis grown at 37°C and pH 7 had equal contributions from Ail and Pla but an undetectable role for Psa. At 37°C and pH 6, both Ail and Psa contributed to binding and Yop delivery, while Pla contributed minimally. Pla-mediated Yop translocation was independent of protease activity. Of the three single mutants, the Δail mutant was the most defective in mouse virulence. The expression level of ail was also the highest of the three adhesins in infected mouse tissues. Compared to an ail mutant, additional deletion of psaA (encoding Psa) led to a 130,000-fold increase in the 50% lethal dose for mice relative to that of the KIM5 parental strain. Our results indicate that in addition to Ail, Pla and Psa can serve as environmentally specific adhesins to facilitate Yop secretion, a critical virulence function of Y. pestis.
Figures




References
-
- Anisimov, A. P., I. V. Bakhteeva, E. A. Panfertsev, T. E. Svetoch, T. B. Kravchenko, M. E. Platonov, G. M. Titareva, T. I. Kombarova, S. A. Ivanov, A. V. Rakin, K. K. Amoako, and S. V. Dentovskaya. 2009. The subcutaneous inoculation of pH 6 antigen mutants of Yersinia pestis does not affect virulence and immune response in mice. J. Med. Microbiol. 58:26-36. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

