The RNA recognition motif of eukaryotic translation initiation factor 3g (eIF3g) is required for resumption of scanning of posttermination ribosomes for reinitiation on GCN4 and together with eIF3i stimulates linear scanning
- PMID: 20679478
- PMCID: PMC2950517
- DOI: 10.1128/MCB.00430-10
The RNA recognition motif of eukaryotic translation initiation factor 3g (eIF3g) is required for resumption of scanning of posttermination ribosomes for reinitiation on GCN4 and together with eIF3i stimulates linear scanning
Abstract
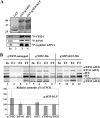
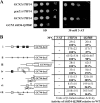
Recent reports have begun unraveling the details of various roles of individual eukaryotic translation initiation factor 3 (eIF3) subunits in translation initiation. Here we describe functional characterization of two essential Saccharomyces cerevisiae eIF3 subunits, g/Tif35 and i/Tif34, previously suggested to be dispensable for formation of the 48S preinitiation complexes (PICs) in vitro. A triple-Ala substitution of conserved residues in the RRM of g/Tif35 (g/tif35-KLF) or a single-point mutation in the WD40 repeat 6 of i/Tif34 (i/tif34-Q258R) produces severe growth defects and decreases the rate of translation initiation in vivo without affecting the integrity of eIF3 and formation of the 43S PICs in vivo. Both mutations also diminish induction of GCN4 expression, which occurs upon starvation via reinitiation. Whereas g/tif35-KLF impedes resumption of scanning for downstream reinitiation by 40S ribosomes terminating at upstream open reading frame 1 (uORF1) in the GCN4 mRNA leader, i/tif34-Q258R prevents full GCN4 derepression by impairing the rate of scanning of posttermination 40S ribosomes moving downstream from uORF1. In addition, g/tif35-KLF reduces processivity of scanning through stable secondary structures, and g/Tif35 specifically interacts with Rps3 and Rps20 located near the ribosomal mRNA entry channel. Together these results implicate g/Tif35 and i/Tif34 in stimulation of linear scanning and, specifically in the case of g/Tif35, also in proper regulation of the GCN4 reinitiation mechanism.
Figures






References
-
- Ahlemann, M., R. Zeidler, S. Lang, B. Mack, M. Münz, and O. Gires. 2006. Carcinoma-associated eIF3i overexpression facilitates mTOR-dependent growth transformation. Mol. Carcinog. 45:957-967. - PubMed
-
- Asano, K., L. Phan, J. Anderson, and A. G. Hinnebusch. 1998. Complex formation by all five homologues of mammalian translation initiation factor 3 subunits from yeast Saccharomyces cerevisiae. J. Biol. Chem. 273:18573-18585. - PubMed
-
- Asano, K., H.-P. Vornlocher, N. J. Richter-Cook, W. C. Merrick, A. G. Hinnebusch, and J. W. B. Hershey. 1997. Structure of cDNAs encoding human eukaryotic initiation factor 3 subunits: possible roles in RNA binding and macromolecular assembly. J. Biol. Chem. 272:27042-27052. - PubMed
-
- Berthelot, K., M. Muldoon, L. Rajkowitsch, J. Hughes, and J. E. G. McCarthy. 2004. Dynamics and processivity of 40S ribosome scanning on mRNA in yeast. Mol. Microbiol. 51:987-1001. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
