VRK2 inhibits mitogen-activated protein kinase signaling and inversely correlates with ErbB2 in human breast cancer
- PMID: 20679487
- PMCID: PMC2950518
- DOI: 10.1128/MCB.01581-09
VRK2 inhibits mitogen-activated protein kinase signaling and inversely correlates with ErbB2 in human breast cancer
Abstract
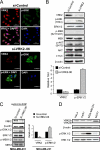
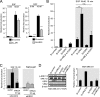
The epidermal growth factor (EGF)-ErbB-mitogen-activated protein kinase (MAPK) transcription signaling pathway is altered in many types of carcinomas, and this pathway can be regulated by new protein-protein interactions. Vaccinia-related kinase (VRK) proteins are Ser-Thr kinases that regulate several signal transduction pathways. In this work, we study the effect of VRK2 on MAPK signaling using breast cancer as a model. High levels of VRK2 inhibit EGF and ErbB2 activation of transcription by the serum response element (SRE). This effect is also detected in response to H-Ras(G12V) or B-Raf(V600E) oncogenes and is accompanied by a reduction in phosphorylated extracellular signal-regulated kinase (ERK) levels, p90RSK levels, and SRE-dependent transcription. Furthermore, VRK2 knockdown has the opposite effect, increasing the transcriptional response to stimulation with EGF and leading to increased levels of ERK phosphorylation. The molecular mechanism lies between MAPK/ERK kinase (MEK) and ERK, since MEK remains phosphorylated while ERK phosphorylation is blocked by VRK2A. This inhibition of the ERK signaling pathway is a consequence of a direct protein-protein interaction between VRK2A, MEK, and kinase suppressor of Ras 1 (KSR1). Identification of new correlations in human cancer can lead to a better understanding of the biology of individual tumors. ErbB2 and VRK2 protein levels were inversely correlated in 136 cases of human breast carcinoma. In ErbB2(+) tumors, there is a significant reduction in the VRK2 level, suggesting a role for VRK2A in ErbB2-MAPK signaling. Thus, VRK2 downregulation in carcinomas permits signal transmission through the MEK-ERK pathway without affecting AKT signaling, causing a signal imbalance among pathways that contributes to the phenotype of breast cancer.
Figures




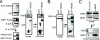

References
-
- Albanese, C., J. Johnson, G. Watanabe, N. Eklund, D. Vu, A. Arnold, and R. G. Pestell. 1995. Transforming p21ras mutants and c-Ets-2 activate the cyclin D1 promoter through distinguishable regions. J. Biol. Chem. 270:23589-23597. - PubMed
-
- Amado, R. G., M. Wolf, M. Peeters, E. Van Cutsem, S. Siena, D. J. Freeman, T. Juan, R. Sikorski, S. Suggs, R. Radinsky, S. D. Patterson, and D. D. Chang. 2008. Wild-type KRAS is required for panitumumab efficacy in patients with metastatic colorectal cancer. J. Clin. Oncol. 26:1626-1634. - PubMed
-
- Benachenhou, N., S. Guiral, I. Gorska-Flipot, D. Labuda, and D. Sinnett. 1998. High resolution deletion mapping reveals frequent allelic losses at the DNA mismatch repair loci hMLH1 and hMSH3 in non-small cell lung cancer. Int. J. Cancer 77:173-180. - PubMed
-
- Blanco, S., L. Klimcakova, F. M. Vega, and P. A. Lazo. 2006. The subcellular localization of vaccinia-related kinase-2 (VRK2) isoforms determines their different effect on p53 stability in tumour cell lines. FEBS J. 273:2487-2504. - PubMed
-
- Blanco, S., and P. A. Lazo. 2009. Vaccinia-related kinase-2. UCSD-Nature Molecule Page doi: 10.1038/mp.a000905.01. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous
