A randomized add-on trial of an N-methyl-D-aspartate antagonist in treatment-resistant bipolar depression
- PMID: 20679587
- PMCID: PMC3000408
- DOI: 10.1001/archgenpsychiatry.2010.90
A randomized add-on trial of an N-methyl-D-aspartate antagonist in treatment-resistant bipolar depression
Abstract
Context: Existing therapies for bipolar depression have a considerable lag of onset of action. Pharmacological strategies that produce rapid antidepressant effects-for instance, within a few hours or days-would have an enormous impact on patient care and public health.
Objective: To determine whether an N-methyl-D-aspartate-receptor antagonist produces rapid antidepressant effects in subjects with bipolar depression.
Design: A randomized, placebo-controlled, double-blind, crossover, add-on study conducted from October 2006 to June 2009.
Setting: Mood Disorders Research Unit at the National Institute of Mental Health, Bethesda, Maryland. Patients Eighteen subjects with DSM-IV bipolar depression (treatment-resistant).
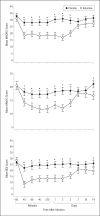
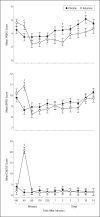
Interventions: Subjects maintained at therapeutic levels of lithium or valproate received an intravenous infusion of either ketamine hydrochloride (0.5 mg/kg) or placebo on 2 test days 2 weeks apart. The Montgomery-Asberg Depression Rating Scale was used to rate subjects at baseline and at 40, 80, 110, and 230 minutes and on days 1, 2, 3, 7, 10, and 14 postinfusion.
Main outcome measures: Change in Montgomery-Asberg Depression Rating Scale primary efficacy measure scores.
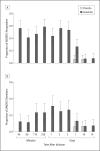
Results: Within 40 minutes, depressive symptoms significantly improved in subjects receiving ketamine compared with placebo (d = 0.52, 95% confidence interval [CI], 0.28-0.76); this improvement remained significant through day 3. The drug difference effect size was largest at day 2 (d = 0.80, 95% CI, 0.55-1.04). Seventy-one percent of subjects responded to ketamine and 6% responded to placebo at some point during the trial. One subject receiving ketamine and 1 receiving placebo developed manic symptoms. Ketamine was generally well tolerated; the most common adverse effect was dissociative symptoms, only at the 40-minute point.
Conclusion: In patients with treatment-resistant bipolar depression, robust and rapid antidepressant effects resulted from a single intravenous dose of an N-methyl-D-aspartate antagonist.
Trial registration: ClinicalTrials.gov NCT00088699.
Figures

Comment in
-
N-methyl-D-aspartate glutamate receptor antagonists and the promise of rapid-acting antidepressants.Arch Gen Psychiatry. 2010 Nov;67(11):1110-1. doi: 10.1001/archgenpsychiatry.2010.138. Arch Gen Psychiatry. 2010. PMID: 21041611 No abstract available.
References
-
- Calabrese JR, Huffman RF, White RL, Edwards S, Thompson TR, Ascher JA, Monaghan ET, Leadbetter RA. Lamotrigine in the acute treatment of bipolar depression: results of five double-blind, placebo-controlled clinical trials. Bipolar Disord. 2008;10(2):323–333. - PubMed
-
- Sachs GS, Nierenberg AA, Calabrese JR, Marangell LB, Wisniewski SR, Gyulai L, Friedman ES, Bowden CL, Fossey MD, Ostacher MJ, Ketter TA, Patel J, Hauser P, Rapport D, Martinez JM, Allen MH, Miklowitz DJ, Otto MW, Dennehy EB, Thase ME. Effectiveness of adjunctive antidepressant treatment for bipolar depression. N Engl J Med. 2007;356(17):1711–1722. - PubMed
-
- Nierenberg AA, Ostacher MJ, Calabrese JR, Ketter TA, Marangell LB, Miklowitz DJ, Miyahara S, Bauer MS, Thase ME, Wisniewski SR, Sachs GS. Treatment-resistant bipolar depression: a STEP-BD equipoise randomized effectiveness trial of antidepressant augmentation with lamotrigine, inositol, or risperidone. Am J Psychiatry. 2006;163(2):210–216. - PubMed
-
- Weisler RH, Calabrese JR, Thase ME, Arvekvist R, Stening G, Paulsson B, Suppes T. Efficacy of quetiapine monotherapy for the treatment of depressive episodes in bipolar I disorder: a post hoc analysis of combined results from 2 double-blind, randomized, placebo-controlled studies. J Clin Psychiatry. 2008;69(5):769–782. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

