Glucarpidase, leucovorin, and thymidine for high-dose methotrexate-induced renal dysfunction: clinical and pharmacologic factors affecting outcome
- PMID: 20679598
- PMCID: PMC2940396
- DOI: 10.1200/JCO.2009.25.4540
Glucarpidase, leucovorin, and thymidine for high-dose methotrexate-induced renal dysfunction: clinical and pharmacologic factors affecting outcome
Abstract
Purpose: To assess the role of the recombinant bacterial enzyme, glucarpidase (carboxypeptidase-G(2)), leucovorin, and thymidine in the management and outcome of patients with high-dose methotrexate (HDMTX) -induced nephrotoxicity.
Methods: Patients with HDMTX-induced nephrotoxicity received one to three doses of intravenous (IV) glucarpidase and leucovorin rescue. The initial cohort (n = 35) also received thymidine by continuous IV infusion. Subsequently, thymidine was restricted to patients with prolonged exposure (> 96 hours) to methotrexate (MTX) or with substantial MTX toxicity at study entry. Plasma MTX, leucovorin, and 5-methyltetrahydrofolate (5-mTHF) concentrations were measured pre- and postglucarpidase. Toxicities were monitored, and logistic regression analysis was used to assess the relationship of baseline characteristics to the development of severe toxicity and death.
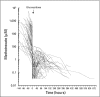
Results: Glucarpidase was administered at a median of 96 hours (receiving thymidine, n = 44) and 66 hours (not receiving thymidine, n = 56) after the start of the MTX infusion. Plasma MTX concentrations decreased within 15 minutes of glucarpidase by 98.7%. Plasma 5-mTHF concentrations also decreased more than 98% after administration of glucarpidase. Of 12 deaths, six were directly attributed to irreversible MTX toxicity. Presence of grade 4 toxicity before administration of glucarpidase, inadequate initial increase in leucovorin dosing, and administration of glucarpidase more than 96 hours after the start of the MTX infusion were associated with development of grade 4 and 5 toxicity.
Conclusion: Early intervention with the combination of leucovorin and glucarpidase is highly effective in patients who develop HDMTX-induced renal dysfunction. Severe toxicity and mortality occurred in patients in whom glucarpidase rescue was delayed and occurred despite thymidine administration.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Figures

Comment in
-
High-dose methotrexate-induced renal dysfunction: is glucarpidase necessary for rescue?J Clin Oncol. 2011 Mar 1;29(7):e180; author reply e181. doi: 10.1200/JCO.2010.32.8245. Epub 2011 Jan 10. J Clin Oncol. 2011. PMID: 21220601 No abstract available.
References
-
- Bleyer WA. Methotrexate: Clinical pharmacology, current status and therapeutic guidelines. Cancer Treat Rev. 1977;4:87–101. - PubMed
-
- Djerassi I. High-dose methotrexate (NSC-740) and citrovorum factor (NSC-3590) rescue: Background and rationale. Cancer Chemother Rep. 1975;6:3–6.
-
- Schwartz S, Borner K, Müller K, et al. Glucarpidase (carboxypeptidase g2) intervention in adult and elderly cancer patients with renal dysfunction and delayed methotrexate elimination after high-dose methotrexate therapy. Oncologist. 2007;12:1299–1308. - PubMed
-
- Widemann BC, Balis FM, Murphy RF, et al. Carboxypeptidase-G2, thymidine, and leucovorin rescue in cancer patients with methotrexate-induced renal dysfunction. J Clin Oncol. 1997;15:2125–2134. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

