Direct molecular analysis of whole-body animal tissue sections by MALDI imaging mass spectrometry
- PMID: 20680598
- PMCID: PMC6905621
- DOI: 10.1007/978-1-60761-746-4_17
Direct molecular analysis of whole-body animal tissue sections by MALDI imaging mass spectrometry
Abstract
The determination of the localization of various compounds in a whole animal is valuable for many applications, including pharmaceutical absorption, distribution, metabolism, and excretion (ADME) studies and biomarker discovery. Imaging mass spectrometry is a powerful tool for localizing compounds of biological interest with molecular specificity and relatively high resolution. Utilizing imaging mass spectrometry for whole-body animal sections offers considerable analytical advantages compared to traditional methods, such as whole-body autoradiography, but the experiment is not straightforward. This chapter addresses the advantages and unique challenges that the application of imaging mass spectrometry to whole-body animal sections entails, including discussions of sample preparation, matrix application, signal normalization, and image generation. Lipid and protein images obtained from whole-body tissue sections of mouse pups are presented along with detailed protocols for the experiments.
Figures



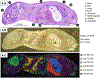
References
-
- Schwartz SA, Reyzer ML, Caprioli RM (2003) Direct tissue analysis using matrix-assisted laser desorption/ionization mass spectrometry: practical aspects of sample preparation. J Mass Spectrom, 38, 699–708 - PubMed
-
- Khatib-Shahidi S, Andersson M, Herman JL, Gillespie TA, and Caprioli RM (2006) Direct molecular analysis of whole-body animal tissue sections by imaging MALDI mass spectrometry. Anal Chem, 78,6448–6456 - PubMed
-
- Nissanov J, Bertrand L, Tretiak O (2001) Cryosectioning distortion reduction using tape support. Microsc Res Tech, 53, 239–240 - PubMed
-
- Chaurand R, Schwartz SA, Billheimer D, Xu BJ, Crecelius A, Caprioli RM (2004) Integrating histology and imaging mass spectrometry. Anal Chem, 76, 1145–1155 - PubMed
-
- Chaurand R, Caprioli RM (2002) Direct profiling and imaging of peptides and proteins from mammalian cells and tissue sections by mass spectrometry. Electrophoresis, 23, 3125–3135 - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

