Analysis of relationships between solid-state properties, counterion, and developability of pharmaceutical salts
- PMID: 20680707
- PMCID: PMC2974123
- DOI: 10.1208/s12249-010-9499-4
Analysis of relationships between solid-state properties, counterion, and developability of pharmaceutical salts
Abstract
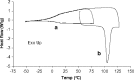
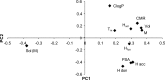
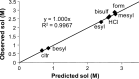
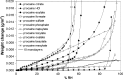
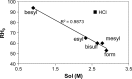
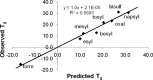
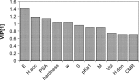
The solid-state properties of pharmaceutical salts, which are dependent on the counterion used to form the salt, are critical for successful development of a stable dosage form. In order to better understand the relationship between counterion and salt properties, 11 salts of procaine, which is a base, were synthesized and characterized using a variety of experimental and computational methods. Correlations between the various experimental and calculated physicochemical properties of the salts and counterions were probed. In addition to investigating the key factors affecting solubility, the hygroscopicity of the crystalline salts was studied to determine which solid-state and counterion properties might be responsible for enhancements in moisture uptake, thus providing the potential for adverse chemical stability. Multivariate principal components and partial least squares projection to latent structures analyses were performed in an attempt to establish predictive models capable of describing the relationships between these characteristics and both measured and calculated properties of the counterion and salt. Some success was achieved with respect to modeling crystalline salt solubility and the glass transition temperature of the amorphous salts. Through the modeling, insight into the relative importance of various descriptors on salt properties was achieved. The solid-state properties of crystalline and amorphous salts of procaine are highly dependent on the nature of the counterion. Important properties including aqueous solubility, melting point, hygroscopicity, and glass transition temperature were found to vary considerably between the different salts.
Figures


References
-
- Gould PL. Salt selection for basic drugs. Int J Pharm. 1986;33(1–3):201–217. doi: 10.1016/0378-5173(86)90055-4. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

