Tailoring DNA structure to increase target hybridization kinetics on surfaces
- PMID: 20681682
- PMCID: PMC3918422
- DOI: 10.1021/ja104859j
Tailoring DNA structure to increase target hybridization kinetics on surfaces
Erratum in
- J Am Chem Soc. 2010 Nov 17;132(45):16296
Abstract
We report a method for increasing the rate of target hybridization on DNA-functionalized surfaces using a short internal complement DNA (sicDNA) strand. The sicDNA causes up to a 5-fold increase in association rate by inducing a conformational change that extends the DNA away from the surface, making it more available to bind target nucleic acids. The sicDNA-induced kinetic enhancement is a general phenomenon that occurred with all sequences and surfaces investigated. Additionally, the process is selective and can be used in multicomponent systems to controllably and orthogonally "turn on" specific sequences by the addition of the appropriate sicDNA. Finally, we show that sicDNA is compatible with systems used in gene regulation, intracellular detection, and microarrays, suggesting several potential therapeutic, diagnostic, and bioinformatic applications.
Figures

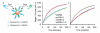


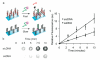
References
-
- Wang K, Tang Z, Yang CJ, Kim Y, Fang X, Li W, Wu Y, Medley CD, Cao Z, Li J, Colon P, Lin H, Tan W. Angew. Chem., Int. Ed. 2009;48:856–870. - PMC - PubMed
- Moses S, Brewer SH, Lowe LB, Lappi SE, Gilvey LBG, Sauthier M, Tenent RC, Feldheim DL, Franzen S. Langmuir. 2004;20:11134–11140. - PubMed
- Katz E, Willner I. Angew. Chem., Int. Ed. 2004;43:6042–6108. - PubMed
- Rosi NL, Mirkin CA. Chem. Rev. 2005;105:1547–1562. - PubMed
- Bath J, Turberfield AJ. Nat. Nanotechnol. 2007;2:275–284. - PubMed
-
- Seelig G, Yurke B, Winfree E. J. Am. Chem. Soc. 2006;128:12211–12220. - PubMed
- Wei HR, Kuan PF, Tian SL, Yang CH, Nie J, Sengupta S, Ruotti V, Jonsdottir GA, Keles S, Thomson JA, Stewart R. Nucleic Acids Res. 2008;36:2926–2938. - PMC - PubMed
- Gao Y, Wolf LK, Georgiadis RM. Nucleic Acids Res. 2006;34:3370–3377. - PMC - PubMed
- Wang YF, Zhang Y, Ong NP. Phys. Rev. E. 2005;72:051918. - PubMed
- Leunissen ME, Dreyfus R, Cheong FC, Grier DG, Sha R, Seeman NC, Chaikin PM. Nat. Mater. 2009;8:590–595. - PubMed
-
- Riccelli PV, Merante F, Leung KT, Bortolin S, Zastawny RL, Janeczko R, Benight AS. Nucleic Acids Res. 2001;29:996–1004. - PMC - PubMed
- Yuan BF, Zhuang XY, Hao YH, Tan Z. Chem. Commun. 2008:6600–6602. - PubMed
- Vasiliskov VA, Prokopenko DV, Mirzabekov AD. Nucleic Acids Res. 2001;29:2303–2313. - PMC - PubMed
- O’Meara D, Nilsson P, Nygren PA, Uhlen M, Lundeberg J. Anal. Biochem. 1998;255:195–203. - PubMed
-
- Maye MM, Nykypanchuk D, van der Lelie D, Gang O. J. Am. Chem. Soc. 2006;128:14020–14021. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

