Single-molecule observation of protein adsorption onto an inorganic surface
- PMID: 20681715
- PMCID: PMC2917251
- DOI: 10.1021/ja1026858
Single-molecule observation of protein adsorption onto an inorganic surface
Abstract
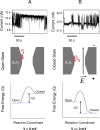
Understanding the interactions between silicon-based materials and proteins from the bloodstream is of key importance in a myriad of realms, such as the design of nanofluidic devices and functional biomaterials, biosensors, and biomedical molecular diagnosis. By using nanopores fabricated in 20 nm-thin silicon nitride membranes and highly sensitive electrical recordings, we show single-molecule observation of nonspecific protein adsorption onto an inorganic surface. A transmembrane potential was applied across a single nanopore-containing membrane immersed into an electrolyte-filled chamber. Through the current fluctuations measured across the nanopore, we detected long-lived captures of bovine serum albumin (BSA), a major multifunctional protein present in the circulatory system. Based upon single-molecule electrical signatures observed in this work, we judge that the bindings of BSA to the nitride surface occurred in two distinct orientations. With some adaptation and further experimentation, this approach, applied on a parallel array of synthetic nanopores, holds potential for use in methodical quantitative studies of protein adsorption onto inorganic surfaces.
Figures




Similar articles
-
Molecular transport of proteins through nanoporous membranes fabricated by interferometric lithography.Phys Chem Chem Phys. 2013 Jan 21;15(3):965-71. doi: 10.1039/c2cp43400h. Phys Chem Chem Phys. 2013. PMID: 23211956
-
Protein-repellent silicon nitride surfaces: UV-induced formation of oligoethylene oxide monolayers.ACS Appl Mater Interfaces. 2011 Mar;3(3):697-704. doi: 10.1021/am100985c. Epub 2011 Feb 10. ACS Appl Mater Interfaces. 2011. PMID: 21309535
-
Detailed Study of BSA Adsorption on Micro- and Nanocrystalline Diamond/β-SiC Composite Gradient Films by Time-Resolved Fluorescence Microscopy.Langmuir. 2017 Jan 24;33(3):802-813. doi: 10.1021/acs.langmuir.6b04177. Epub 2017 Jan 9. Langmuir. 2017. PMID: 28025889
-
Effect of pH on the adsorption and interactions of Bovine Serum Albumin with functionalized silicon nitride surface.Colloids Surf B Biointerfaces. 2018 Jul 1;167:441-447. doi: 10.1016/j.colsurfb.2018.03.045. Epub 2018 Mar 29. Colloids Surf B Biointerfaces. 2018. PMID: 29709828
-
Characterization of protein unfolding with solid-state nanopores.Protein Pept Lett. 2014 Mar;21(3):256-65. doi: 10.2174/09298665113209990077. Protein Pept Lett. 2014. PMID: 24370259 Free PMC article. Review.
Cited by
-
Tuning Contact Angles of Aqueous Droplets on Hydrophilic and Hydrophobic Surfaces by Surfactants.J Phys Chem B. 2022 May 5;126(17):3374-3384. doi: 10.1021/acs.jpcb.2c01599. Epub 2022 Apr 25. J Phys Chem B. 2022. PMID: 35468298 Free PMC article.
-
Electrically facilitated translocation of protein through solid nanopore.Nanoscale Res Lett. 2014 Mar 24;9(1):140. doi: 10.1186/1556-276X-9-140. Nanoscale Res Lett. 2014. PMID: 24661490 Free PMC article.
-
Fine-tuning the size and minimizing the noise of solid-state nanopores.J Vis Exp. 2013 Oct 31;(80):e51081. doi: 10.3791/51081. J Vis Exp. 2013. PMID: 24300128 Free PMC article.
-
Insights on using plastic-based dual in-plane nanopore sensors for differentiation and shape determinations of single protein molecules.Sci Rep. 2025 Apr 21;15(1):13742. doi: 10.1038/s41598-025-96232-y. Sci Rep. 2025. PMID: 40258844 Free PMC article.
-
Single-Entity Detection With TEM-Fabricated Nanopores.Front Chem. 2021 May 7;9:664820. doi: 10.3389/fchem.2021.664820. eCollection 2021. Front Chem. 2021. PMID: 34026729 Free PMC article. Review.
References
-
- Roach P, Farrar D, Perry CC. J. Am. Chem. Soc. 2006;128:3939–3945. - PubMed
-
- Roach P, Farrar D, Perry CC. J. Am. Chem. Soc. 2005;127:8168–8173. - PubMed
-
- Brewer SH, Glomm WR, Johnson MC, Knag MK, Franzen S. Langmuir. 2005;21:9303–9307. - PubMed
-
- Rabe M, Verdes D, Zimmermann J, Seeger S. J. Phys. Chem. B. 2008;112:13971–13980. - PubMed
-
- Rabe M, Verdes D, Rankl M, Artus GR, Seeger S. Chemphyschem. 2007;8:862–872. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

