New insights into poly(lactic-co-glycolic acid) microstructure: using repeating sequence copolymers to decipher complex NMR and thermal behavior
- PMID: 20681726
- PMCID: PMC3432321
- DOI: 10.1021/ja102670n
New insights into poly(lactic-co-glycolic acid) microstructure: using repeating sequence copolymers to decipher complex NMR and thermal behavior
Abstract
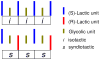
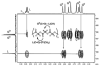
Sequence, which Nature uses to spectacular advantage, has not been fully exploited in synthetic copolymers. To investigate the effect of sequence and stereosequence on the physical properties of copolymers, a family of complex isotactic, syndiotactic, and atactic repeating sequence poly(lactic-co-glycolic acid) copolymers (RSC PLGAs) were prepared and their NMR and thermal behavior was studied. The unique suitability of polymers prepared from the bioassimilable lactic and glycolic acid monomers for biomedical applications makes them ideal candidates for this type of sequence engineering. Polymers with repeating units of LG, GLG and LLG (L = lactic, G = glycolic) with controlled and varied tacticities were synthesized by assembly of sequence-specific, stereopure dimeric, trimeric, and hexameric segmer units. Specifically labeled deuterated lactic and glycolic acid segmers were likewise prepared and polymerized. Molecular weights for the copolymers were in the range M(n) = 12-40 kDa by size exclusion chromatography in THF. Although the effects of sequence-influenced solution conformation were visible in all resonances of the (1)H and (13)C NMR spectra, the diastereotopic methylene resonances in the (1)H NMR (CDCl(3)) for the glycolic units of the copolymers proved most sensitive. An octad level of resolution, which corresponds to an astounding 31-atom distance between the most separated stereocenters, was observed in some mixed sequence polymers. Importantly, the level of sensitivity of a particular NMR resonance to small differences in sequence was found to depend on the sequence itself. Thermal properties were also correlated with sequence.
Figures



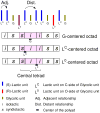
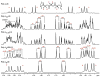


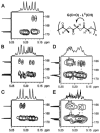
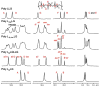

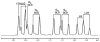
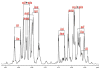
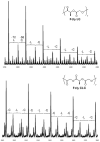
 ” central tetrad and exhibit similar chemical shifts, the shifts of the nearly hexad-resolved sequences create a pattern that does not show an easily interpretable correspondence with the standard.
” central tetrad and exhibit similar chemical shifts, the shifts of the nearly hexad-resolved sequences create a pattern that does not show an easily interpretable correspondence with the standard.

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

