T cells in multiple sclerosis and experimental autoimmune encephalomyelitis
- PMID: 20682002
- PMCID: PMC2990924
- DOI: 10.1111/j.1365-2249.2010.04143.x
T cells in multiple sclerosis and experimental autoimmune encephalomyelitis
Abstract
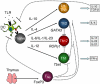
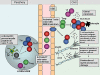
Multiple sclerosis (MS) is a demyelinating inflammatory disorder of the central nervous system (CNS), which involves autoimmune responses to myelin antigens. Studies in experimental autoimmune encephalomyelitis (EAE), an animal model for MS, have provided convincing evidence that T cells specific for self-antigens mediate pathology in these diseases. Until recently, T helper type 1 (Th1) cells were thought to be the main effector T cells responsible for the autoimmune inflammation. However more recent studies have highlighted an important pathogenic role for CD4(+) T cells that secrete interleukin (IL)-17, termed Th17, but also IL-17-secreting γδ T cells in EAE as well as other autoimmune and chronic inflammatory conditions. This has prompted intensive study of the induction, function and regulation of IL-17-producing T cells in MS and EAE. In this paper, we review the contribution of Th1, Th17, γδ, CD8(+) and regulatory T cells as well as the possible development of new therapeutic approaches for MS based on manipulating these T cell subtypes.
© 2010 The Authors. Clinical and Experimental Immunology © 2010 British Society for Immunology.
Figures
References
-
- Frohman EM, Racke MK, Raine CS. Multiple sclerosis – the plaque and its pathogenesis. N Engl J Med. 2006;354:942–55. - PubMed
-
- Compston A, Coles A. Multiple sclerosis. Lancet. 2008;372:1502–17. - PubMed
-
- McDonald WI, Sears TA. The effects of experimental demyelination on conduction in the central nervous system. Brain. 1970;93:583–98. - PubMed
-
- Polman CH, O'Connor PW, Havrdova E, et al. A randomized, placebo-controlled trial of natalizumab for relapsing multiple sclerosis. N Engl J Med. 2006;354:899–910. - PubMed
-
- Stromnes IM, Goverman JM. Active induction of experimental allergic encephalomyelitis. Nat Protoc. 2006;1:1810–19. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

