Type 1 diabetes predisposes to enhanced gingival leukocyte margination and macromolecule extravasation in vivo
- PMID: 20682016
- PMCID: PMC3501126
- DOI: 10.1111/j.1600-0765.2010.01295.x
Type 1 diabetes predisposes to enhanced gingival leukocyte margination and macromolecule extravasation in vivo
Abstract
Background and objective: Diabetes predisposes to periodontal disease. However, the cellular and molecular mechanisms linking the two conditions are not clear. The impact of chronic hyperglycemia on leukocyte margination and macromolecule extravasation was determined in gingival vessels in vivo.
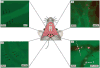
Materials and methods: Gingival intravital microscopy was employed to measure extravasation of fluorescein isothiocyanate (FITC)-dextran in diabetic Akita and healthy wild-type (WT) mice. Rhodamine 6G and FITC-LY6G were injected for nonspecific and polymorphonuclear-specific leukocyte labeling, respectively. Surface expression of leukocyte adhesion molecules was determined with flow cytometry and western blotting.
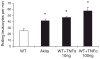
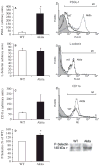
Results: Vascular permeability was significantly increased in Akita gingival vessels compared with WT [permeability index (PI): WT, 0.75 ± 0.05; Akita, 1.1 ± 0.03: p < 0.05). Wild-type gingival vessels reached comparable permeability 2 h after intragingival injection of tumor necrosis factor α (TNFα), used here as positive control (PI, 1.17 ± 0.16). The number of rolling leukocytes was significantly elevated in diabetic gingiva (WT, 25 ± 3.7 cells/min; Akita, 42 ± 8.5 cells/min; p < 0.03). Similar rolling cell counts were obtained in WT after intragingival injection of TNFα (10 ng TNFα, 47 ± 1.3 cells/min; 100 ng TNFα, 57.5 ± 5.85 cells/min). The number of leukocytes firmly attached to the endothelium was similar in WT and Akita mice. Leukocyte cell-surface expression of P-selectin glycoprotein ligand-1 and CD11a was increased in Akita mice, while L-selectin remained unchanged when compared with WT. Moreover, P-selectin expression in Akita gingival tissues was elevated compared with that of WT.
Conclusion: Chronic hyperglycemia induces a proinflammatory state in the gingival microcirculation characterized by increased vascular permeability, and leukocyte and endothelial cell activation. Leukocyte-induced microvascular damage, in turn, may contribute to periodontal tissue damage in diabetes.
© 2010 John Wiley & Sons A/S.
Figures


Similar articles
-
Chronic hyperglycemia predisposes to exaggerated inflammatory response and leukocyte dysfunction in Akita mice.J Immunol. 2006 Nov 15;177(10):7250-6. doi: 10.4049/jimmunol.177.10.7250. J Immunol. 2006. PMID: 17082643 Free PMC article.
-
Reduced blood brain barrier breakdown in P-selectin deficient mice following transient ischemic stroke: a future therapeutic target for treatment of stroke.BMC Neurosci. 2010 Feb 2;11:12. doi: 10.1186/1471-2202-11-12. BMC Neurosci. 2010. PMID: 20122276 Free PMC article.
-
Gingival inflammation and leukocyte-endothelium cell interactions in women with polycystic ovary syndrome.J Periodontol. 2025 May;96(5):478-489. doi: 10.1002/JPER.24-0148. Epub 2024 Oct 15. J Periodontol. 2025. PMID: 39403884 Free PMC article.
-
Adhesive Mechanisms of Histone-Induced Neutrophil-Endothelium Interactions in the Muscle Microcirculation.Eur Surg Res. 2016;56(1-2):19-31. doi: 10.1159/000441778. Epub 2015 Nov 18. Eur Surg Res. 2016. PMID: 26575178
-
Spatiotemporal expression dynamics of selectins govern the sequential extravasation of neutrophils and monocytes in the acute inflammatory response.Arterioscler Thromb Vasc Biol. 2015 Apr;35(4):899-910. doi: 10.1161/ATVBAHA.114.305143. Epub 2015 Feb 26. Arterioscler Thromb Vasc Biol. 2015. PMID: 25722429
Cited by
-
Diabetes mellitus and periodontal diseases.Curr Diab Rep. 2013 Jun;13(3):445-52. doi: 10.1007/s11892-013-0367-y. Curr Diab Rep. 2013. PMID: 23430581 Review.
-
Therapeutic Targets for Management of Periodontitis and Diabetes.Curr Pharm Des. 2016;22(15):2216-37. doi: 10.2174/1381612822666160216150338. Curr Pharm Des. 2016. PMID: 26881443 Free PMC article. Review.
-
Management of diabolical diabetes mellitus and periodontitis nexus: Are we doing enough?World J Diabetes. 2016 Feb 25;7(4):50-66. doi: 10.4239/wjd.v7.i4.50. World J Diabetes. 2016. PMID: 26962409 Free PMC article. Review.
-
The role of oral microbiome in periodontitis under diabetes mellitus.J Oral Microbiol. 2022 Jun 3;14(1):2078031. doi: 10.1080/20002297.2022.2078031. eCollection 2022. J Oral Microbiol. 2022. PMID: 35694215 Free PMC article. Review.
-
Evaluating All Potential Oral Complications of Diabetes Mellitus.Front Endocrinol (Lausanne). 2019 Feb 18;10:56. doi: 10.3389/fendo.2019.00056. eCollection 2019. Front Endocrinol (Lausanne). 2019. PMID: 30962800 Free PMC article. Review.
References
-
- Southerland JH, Taylor GW, Moss K, Beck JD, Offenbacher S. Commonality in chronic inflammatory diseases: periodontitis, diabetes, and coronary artery disease. Periodontol 2000. 2006;40:130–143. - PubMed
-
- Haffajee AD, Socransky SS. Microbiology of periodontal diseases: introduction. Periodontol 2000. 2005;38:9–12. - PubMed
-
- Socransky SS, Haffajee AD. Periodontal microbial ecology. Periodontol 2000. 2005;38:135–187. - PubMed
-
- Gapski R, Hasturk H, Van Dyke TE, et al. Systemic MMP inhibition for periodontal wound repair: results of a multi-centre randomized-controlled clinical trial. J Clin Periodontol. 2009;36:149–156. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

