Mortality associated with administration of high-dose tranexamic acid and aprotinin in primary open-heart procedures: a retrospective analysis
- PMID: 20682059
- PMCID: PMC2945131
- DOI: 10.1186/cc9216
Mortality associated with administration of high-dose tranexamic acid and aprotinin in primary open-heart procedures: a retrospective analysis
Abstract
Introduction: Antifibrinolytic agents are commonly used during cardiac surgery to minimize bleeding. Because of safety concerns, aprotinin was withdrawn from the market in 2007. Since then, tranexamic acid (TXA) has become the antifibrinolytic treatment of choice in many heart centers. The safety profile of TXA has not been extensively studied. Therefore, the aim of this study was to evaluate safety and efficiency of TXA compared with aprotinin in cardiac surgery.
Methods: Since July 1, 2006, TXA has been administered at a dose of 50 mg/kg tranexamic acid before cardiopulmonary bypass (CPB) and 50 mg/kg into the priming fluid of the CPB. Prior to this, all patients were treated with aprotinin at a dose of 50,000 KIU per kilogram body weight. Safety was evaluated with mortality, biomarkers, and the diagnosis of myocardial infarction, ischemic stroke, convulsive seizures, and acute renal failure in the intensive care unit (ICU), intermediate care unit (IMCU), and hospital stay. Efficiency was evaluated by the need for transfusion of blood products and total postoperative blood loss.
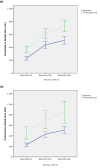
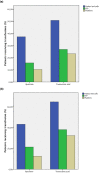
Results: After informed consent, 893 patients were included in our database (557 consecutive patients receiving aprotinin and 336 patients receiving TXA). A subgroup of 320 patients undergoing open-heart procedures (105 receiving TXA and 215 receiving aprotinin) was analyzed separately. In the aprotinin group, a higher rate of late events of ischemic stroke (3.4% versus 0.9%; P = 0.02) and neurologic disability (5.8% versus 2.4%; P = 0.02) was found. The rate of postoperative convulsive seizures was increased in tendency in patients receiving TXA (2.7% versus 0.9%; P = 0.05). The use of TXA was associated with higher cumulative drainage losses (PANOVA < 0.01; Ptime < 0.01) and a higher rate of repeated thoracotomy for bleeding (6.9% versus 2.4%; P < 0.01). In the subgroup of patients with open-chamber procedures, mortality was higher in the TXA group (16.2% TXA versus 7.5% aprotinin; P = 0.02). Multivariate logistic regression identified EURO score II and CPB time as additional risk factors for this increased mortality.
Conclusions: The use of high-dose TXA is questioned, as our data suggest an association between higher mortality and minor efficiency while the safety profile of this drug is not consistently improved. Further confirmatory prospective studies evaluating the efficacy and safety profile of TXA are urgently needed to find a safe dosage for this antifibrinolytic drug.
Figures

Comment in
-
Tranexamic acid in cardiac surgery: is there a cause for concern?Crit Care. 2010;14(5):194. doi: 10.1186/cc9227. Epub 2010 Sep 6. Crit Care. 2010. PMID: 20831841 Free PMC article. Review.
Similar articles
-
The risks associated with aprotinin use: a retrospective study of cardiac cases in Nova Scotia.Can J Anaesth. 2013 Jan;60(1):16-23. doi: 10.1007/s12630-012-9806-5. Epub 2012 Nov 7. Can J Anaesth. 2013. PMID: 23132043
-
Tranexamic acid and aprotinin in primary cardiac operations: an analysis of 220 cardiac surgical patients treated with tranexamic acid or aprotinin.Anesth Analg. 2008 Nov;107(5):1469-78. doi: 10.1213/ane.0b013e318182252b. Anesth Analg. 2008. PMID: 18931201 Clinical Trial.
-
Aprotinin versus tranexamic acid in children undergoing cardiac surgery: an observational study.Eur J Cardiothorac Surg. 2019 Oct 1;56(4):688-695. doi: 10.1093/ejcts/ezz088. Eur J Cardiothorac Surg. 2019. PMID: 30928999
-
Antifibrinolytic Drugs for the Prevention of Bleeding in Pediatric Cardiac Surgery on Cardiopulmonary Bypass: A Systematic Review and Meta-analysis.Anesth Analg. 2022 May 1;134(5):987-1001. doi: 10.1213/ANE.0000000000005760. Anesth Analg. 2022. PMID: 34633994
-
Tranexamic acid: a review of its use in surgery and other indications.Drugs. 1999 Jun;57(6):1005-32. doi: 10.2165/00003495-199957060-00017. Drugs. 1999. PMID: 10400410 Review.
Cited by
-
Recent advances on plasmin inhibitors for the treatment of fibrinolysis-related disorders.Med Res Rev. 2014 Nov;34(6):1168-216. doi: 10.1002/med.21315. Epub 2014 Mar 21. Med Res Rev. 2014. PMID: 24659483 Free PMC article. Review.
-
Treatment of Diffuse Alveolar Hemorrhage: Controlling Inflammation and Obtaining Rapid and Effective Hemostasis.Int J Mol Sci. 2021 Jan 14;22(2):793. doi: 10.3390/ijms22020793. Int J Mol Sci. 2021. PMID: 33466873 Free PMC article. Review.
-
Intra-articular injection of tranexamic acid in patients with haemophilia arthritis: retrospective controlled study in total knee arthroplasty.Int Orthop. 2024 Mar;48(3):683-692. doi: 10.1007/s00264-023-05983-8. Epub 2023 Sep 23. Int Orthop. 2024. PMID: 37740768
-
Safety and effectiveness of two treatment regimes with tranexamic acid to minimize inflammatory response in elective cardiopulmonary bypass patients: a randomized double-blind, dose-dependent, phase IV clinical trial.J Cardiothorac Surg. 2011 Oct 14;6:138. doi: 10.1186/1749-8090-6-138. J Cardiothorac Surg. 2011. PMID: 21999189 Free PMC article. Clinical Trial.
-
Tranexamic acid-associated seizures: Causes and treatment.Ann Neurol. 2016 Jan;79(1):18-26. doi: 10.1002/ana.24558. Epub 2015 Dec 15. Ann Neurol. 2016. PMID: 26580862 Free PMC article. Review.
References
-
- Mangano D, Miao Y, Vuylsteke A, Tudor IC, Juneja R, Filipescu D, Hoeft A, Fontes ML, Hillel Z, Ott E, Titov T, Dietzel C, Levin J. Group IoTMSoPIR, Foundation IRaE. Mortality associated with aprotinin during 5 years following coronary artery bypass graft surgery. JAMA. 2007;297:471–479. doi: 10.1001/jama.297.5.471. - DOI - PubMed
-
- Karkouti K, Beattie WS, Dattilo KM, McCluskey SA, Ghannam M, Hamdy A, Wijeysundera DN, Fedorko L, Yau TM. A propensity score case-control comparison of aprotinin and tranexamic acid in high-transfusion-risk cardiac surgery. Transfusion. 2006;46:327–338. doi: 10.1111/j.1537-2995.2006.00724.x. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

