Rbx1 flexible linker facilitates cullin-RING ligase function before neddylation and after deneddylation
- PMID: 20682250
- PMCID: PMC2913186
- DOI: 10.1016/j.bpj.2010.05.021
Rbx1 flexible linker facilitates cullin-RING ligase function before neddylation and after deneddylation
Abstract
In ubiquitination, cullin-RING E3 ubiquitin ligases (CRLs) assist in ubiquitin transfer from ubiquitin-conjugating enzyme E2 to the substrate. Neddylation, which involves NEDD8 transfer from E2 to E3-cullin, stimulates ubiquitination by inducing conformational change in CRLs. However, deneddylation, which removes NEDD8 from cullin, does not suppress ubiquitination in vivo, raising the question of how neddylation/deneddylation exerts its effects. Using molecular-dynamics simulations, we demonstrate that before neddylation occurs, the linker flexibility of Rbx1, a CRL component, leads to conformational changes in CRLs that allow neddylation and initiation of ubiquitination. These large NEDD8-induced conformational changes are retained after deneddylation, allowing both initiation of the ubiquitination process and ubiquitin chain elongation after deneddylation. Furthermore, mutation of lysine, the cullin residue to which NEDD8 covalently attaches, dramatically reduces CRL conformational changes, suggesting that the acceptor lysine allosterically regulates CRLs. Thus, our results imply that neddylation stimulates ubiquitination by CRL conformational control via lysine modification.
2010 Biophysical Society. Published by Elsevier Inc. All rights reserved.
Figures



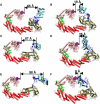
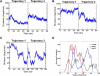
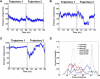
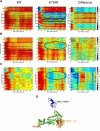
Similar articles
-
Cullin neddylation may allosterically tune polyubiquitin chain length and topology.Biochem J. 2017 Feb 20;474(5):781-795. doi: 10.1042/BCJ20160748. Biochem J. 2017. PMID: 28082425 Free PMC article.
-
Neddylation-induced conformational control regulates cullin RING ligase activity in vivo.J Mol Biol. 2011 Jun 3;409(2):136-45. doi: 10.1016/j.jmb.2011.03.023. Epub 2011 Apr 2. J Mol Biol. 2011. PMID: 21463634
-
Protection of cullin-RING E3 ligases by CSN-UBP12.Trends Cell Biol. 2006 Jul;16(7):362-9. doi: 10.1016/j.tcb.2006.05.001. Epub 2006 Jun 9. Trends Cell Biol. 2006. PMID: 16762551 Review.
-
Modulation of Cullin-RING E3 ubiquitin ligase-dependent ubiquitination by small molecule compounds.J Biol Chem. 2024 Mar;300(3):105752. doi: 10.1016/j.jbc.2024.105752. Epub 2024 Feb 13. J Biol Chem. 2024. PMID: 38354780 Free PMC article.
-
Assembly and Regulation of CRL Ubiquitin Ligases.Adv Exp Med Biol. 2020;1217:33-46. doi: 10.1007/978-981-15-1025-0_3. Adv Exp Med Biol. 2020. PMID: 31898220 Review.
Cited by
-
Stochastic simulation of structural properties of natively unfolded and denatured proteins.J Mol Model. 2012 Sep;18(9):4503-16. doi: 10.1007/s00894-012-1456-6. Epub 2012 May 29. J Mol Model. 2012. PMID: 22643976
-
Autophagy suppresses interleukin-1β (IL-1β) signaling by activation of p62 degradation via lysosomal and proteasomal pathways.J Biol Chem. 2012 Feb 3;287(6):4033-40. doi: 10.1074/jbc.M111.280065. Epub 2011 Dec 13. J Biol Chem. 2012. PMID: 22167182 Free PMC article.
-
High-speed atomic force microscopy directly visualizes conformational dynamics of the HIV Vif protein in complex with three host proteins.J Biol Chem. 2020 Aug 21;295(34):11995-12001. doi: 10.1074/jbc.RA120.014442. Epub 2020 Jun 24. J Biol Chem. 2020. PMID: 32587092 Free PMC article.
-
Advancing targeted protein degradation for cancer therapy.Nat Rev Cancer. 2021 Oct;21(10):638-654. doi: 10.1038/s41568-021-00365-x. Epub 2021 Jun 15. Nat Rev Cancer. 2021. PMID: 34131295 Free PMC article. Review.
-
p97/VCP promotes Cullin-RING-ubiquitin-ligase/proteasome-dependent degradation of IκBα and the preceding liberation of RelA from ubiquitinated IκBα.J Cell Mol Med. 2016 Jan;20(1):58-70. doi: 10.1111/jcmm.12702. Epub 2015 Oct 14. J Cell Mol Med. 2016. PMID: 26463447 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

