Distribution of electromechanical delay in the heart: insights from a three-dimensional electromechanical model
- PMID: 20682251
- PMCID: PMC2913183
- DOI: 10.1016/j.bpj.2010.05.028
Distribution of electromechanical delay in the heart: insights from a three-dimensional electromechanical model
Abstract
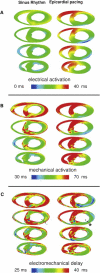
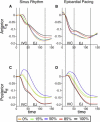
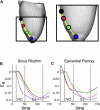
In the intact heart, the distribution of electromechanical delay (EMD), the time interval between local depolarization and myocyte shortening onset, depends on the loading conditions. The distribution of EMD throughout the heart remains, however, unknown because current experimental techniques are unable to evaluate three-dimensional cardiac electromechanical behavior. The goal of this study was to determine the three-dimensional EMD distributions in the intact ventricles for sinus rhythm (SR) and epicardial pacing (EP) by using a new, to our knowledge, electromechanical model of the rabbit ventricles that incorporates a biophysical representation of myofilament dynamics. Furthermore, we aimed to ascertain the mechanisms that underlie the specific three-dimensional EMD distributions. The results revealed that under both conditions, the three-dimensional EMD distribution is nonuniform. During SR, EMD is longer at the epicardium than at the endocardium, and is greater near the base than at the apex. After EP, the three-dimensional EMD distribution is markedly different; it also changes with the pacing rate. For both SR and EP, late-depolarized regions were characterized with significant myofiber prestretch caused by the contraction of the early-depolarized regions. This prestretch delays myofiber-shortening onset, and results in a longer EMD, giving rise to heterogeneous three-dimensional EMD distributions.
2010 Biophysical Society. Published by Elsevier Inc. All rights reserved.
Figures





References
-
- Prinzen F.W., Augustijn C.H., Reneman R.S. The time sequence of electrical and mechanical activation during spontaneous beating and ectopic stimulation. Eur. Heart J. 1992;13:535–543. - PubMed
-
- Badke F.R., Boinay P., Covell J.W. Effects of ventricular pacing on regional left ventricular performance in the dog. Am. J. Physiol. 1980;238:H858–H867. - PubMed
-
- Vetter F.J., McCulloch A.D. Three-dimensional analysis of regional cardiac function: a model of rabbit ventricular anatomy. Prog. Biophys. Mol. Biol. 1998;69:157–183. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

