Atomic force microscopy reveals the alternating subunit arrangement of the TRPP2-TRPV4 heterotetramer
- PMID: 20682256
- PMCID: PMC2913176
- DOI: 10.1016/j.bpj.2010.05.012
Atomic force microscopy reveals the alternating subunit arrangement of the TRPP2-TRPV4 heterotetramer
Abstract
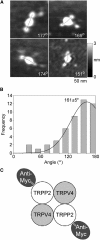
There is evidence that polycystin-2 (TRPP2) interacts with two other members of the transient receptor potential (TRP) family, TRPC1 and TRPV4. We have previously shown that TRPP2 forms a heteromeric complex with TRPC1, with a 2:2 stoichiometry and an alternating subunit arrangement. Here, we used coimmunoprecipitation to show that TRPP2 also interacts with TRPV4, but not with TRPA1 or TRPM8; hence, its promiscuity is limited. We then used atomic force microscopy to study the structure of the TRPV4 homomer and the interaction between TRPP2 and TRPV4. The molecular volume of V5-tagged TRPV4 isolated from singly-transfected tsA 201 cells indicated that it assembled as a homotetramer. The distribution of angles between pairs of anti-V5 antibodies bound to TRPV4 particles had a large peak close to 90 degrees and a smaller peak close to 180 degrees , again consistent with the assembly of TRPV4 as a homotetramer. In contrast, the angle distributions for decoration of the TRPP2-TRPV4 heteromer by either anti-Myc or anti-V5 antibodies had major peaks close to 180 degrees. This result indicates that TRPP2-TRPV4 assembles identically to TRPP2-TRPC1, suggesting a common subunit arrangement among heteromeric TRP channels.
2010 Biophysical Society. Published by Elsevier Inc. All rights reserved.
Figures





References
-
- Mochizuki T., Wu G., Hayashi T., Somlo S. PKD2, a gene for polycystic kidney disease that encodes an integral membrane protein. Science. 1996;272:1339–1342. - PubMed
-
- Hanaoka K., Qian F., Germino G.G. Co-assembly of polycystin-1 and -2 produces unique cation-permeable currents. Nature. 2000;408:990–994. - PubMed
-
- Nauli S.M., Alenghat F.J., Zhou J. Polycystins 1 and 2 mediate mechanosensation in the primary cilium of kidney cells. Nat. Genet. 2003;33:129–137. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

