Receptors with low affinity for neurosteroids and GABA contribute to tonic inhibition of granule cells in epileptic animals
- PMID: 20682339
- PMCID: PMC2940226
- DOI: 10.1016/j.nbd.2010.07.016
Receptors with low affinity for neurosteroids and GABA contribute to tonic inhibition of granule cells in epileptic animals
Abstract
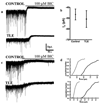
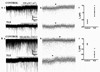
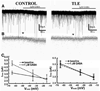
Neurosteroid sensitivity of GABA(A) receptor mediated inhibition of the hippocampal dentate granule cells (DGCs) is reduced in animal models of temporal lobe epilepsy. However, the properties and subunit composition of GABA(A) receptors mediating tonic inhibition in DGCs of epileptic animals have not been described. In the DGCs of epileptic animals, allopregnanolone and L-655708 sensitivity of holding current was diminished and δ subunit was retained in the endoplasmic reticulum and its surface expression was decreased the in the hippocampus. Ro15-4513 and lanthanum had distinct effects on holding current recorded from DGCs of control and epileptic animals suggesting that the pharmacological properties of GABA(A) receptors maintaining tonic inhibition in DGCs of epileptic animals were similar to those containing the α4βxγ2 subunits. Furthermore, surface expression of the α4 subunit increased and a larger fraction of the subunit co-immunoprecipitated with theγ2 subunit in hippocampi of epileptic animals. Together, these studies revealed that functional α4βxδ and α5βxγ2 receptors were reduced in the hippocampi of epileptic animals and that novel α4bxγ2 receptors contributed to the maintenance of tonic inhibition. The presence of α4βxγ2 receptors resulted in low GABA affinity and neurosteroid sensitivity of tonic currents in the DGCs of epileptic animals that could potentially increase seizure vulnerability. These receptors may represent a novel therapeutic target for anticonvulsant drugs without sedative actions.
Copyright © 2010 Elsevier Inc. All rights reserved.
Figures





Similar articles
-
Neurosteroid regulation of GABAA receptors: A role in catamenial epilepsy.Brain Res. 2019 Jan 15;1703:31-40. doi: 10.1016/j.brainres.2018.02.031. Epub 2018 Feb 23. Brain Res. 2019. PMID: 29481795 Free PMC article. Review.
-
Neurosteroid-sensitive δ-GABAA receptors: A role in epileptogenesis?Epilepsia. 2017 Mar;58(3):494-504. doi: 10.1111/epi.13660. Epub 2017 Jan 18. Epilepsia. 2017. PMID: 28452419 Free PMC article.
-
Diminished neurosteroid sensitivity of synaptic inhibition and altered location of the alpha4 subunit of GABA(A) receptors in an animal model of epilepsy.J Neurosci. 2007 Nov 14;27(46):12641-50. doi: 10.1523/JNEUROSCI.4141-07.2007. J Neurosci. 2007. PMID: 18003843 Free PMC article.
-
N-methyl-D-aspartic acid receptor activation downregulates expression of δ subunit-containing GABAA receptors in cultured hippocampal neurons.Mol Pharmacol. 2013 Jul;84(1):1-11. doi: 10.1124/mol.112.084715. Epub 2013 Apr 12. Mol Pharmacol. 2013. PMID: 23585058 Free PMC article.
-
GABA-A Receptors Mediate Tonic Inhibition and Neurosteroid Sensitivity in the Brain.Vitam Horm. 2018;107:177-191. doi: 10.1016/bs.vh.2017.12.001. Epub 2018 Feb 9. Vitam Horm. 2018. PMID: 29544630 Review.
Cited by
-
Seizure-related regulation of GABAA receptors in spontaneously epileptic rats.Neurobiol Dis. 2015 May;77:246-56. doi: 10.1016/j.nbd.2015.03.001. Epub 2015 Mar 11. Neurobiol Dis. 2015. PMID: 25769812 Free PMC article.
-
Decrease in tonic inhibition contributes to increase in dentate semilunar granule cell excitability after brain injury.J Neurosci. 2012 Feb 15;32(7):2523-37. doi: 10.1523/JNEUROSCI.4141-11.2012. J Neurosci. 2012. PMID: 22396425 Free PMC article.
-
Neurosteroid regulation of GABAA receptors: A role in catamenial epilepsy.Brain Res. 2019 Jan 15;1703:31-40. doi: 10.1016/j.brainres.2018.02.031. Epub 2018 Feb 23. Brain Res. 2019. PMID: 29481795 Free PMC article. Review.
-
Calcium-permeable AMPA receptors are expressed in a rodent model of status epilepticus.Ann Neurol. 2012 Jul;72(1):91-102. doi: 10.1002/ana.23570. Ann Neurol. 2012. PMID: 22829271 Free PMC article.
-
Long-Term Effects of Moderate Concussive Brain Injury During Adolescence on Synaptic and Tonic GABA Currents in Dentate Granule Cells and Semilunar Granule Cells.Front Neurosci. 2022 Mar 14;16:800733. doi: 10.3389/fnins.2022.800733. eCollection 2022. Front Neurosci. 2022. PMID: 35360164 Free PMC article.
References
-
- Araujo F, Ruano D, Vitorica J. Absence of association between [delta] and [gamma]2 subunits in native GABAA receptors from rat brain. Eur J of Pharmacol. 1998;347:347–353. - PubMed
-
- Belelli D, Herd MB, Mitchell EA, Peden DR, Vardy AW, Gentet L, Lambert JJ. Neuroactive steroids and inhibitory neurotransmission: mechanisms of action and physiological relevance. Neuroscience. 2006;138:821–829. - PubMed
-
- Bencsits E, Ebert V, Tretter V, Sieghart W. A significant part of native GABAA receptors containing α4 subunits do not contain γ or δ subunits. J Biol Chem. 1999;274:19613–19616. - PubMed
-
- Bertram EH, Williamson JM, Cornett JF, Spradlin S, Chen ZF. Design and construction of a long-term continuous video-EEG monitoring unit for simultaneous recording of multiple small animals. Brain Res Brain Research Protocols. 1997;2:85–97. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

