Adult SVZ lineage cells home to and leave the vascular niche via differential responses to SDF1/CXCR4 signaling
- PMID: 20682445
- PMCID: PMC2916873
- DOI: 10.1016/j.stem.2010.05.019
Adult SVZ lineage cells home to and leave the vascular niche via differential responses to SDF1/CXCR4 signaling
Abstract
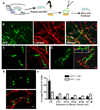
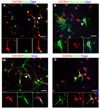
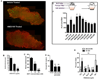
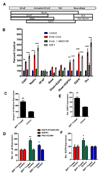
Neural progenitor cells (NPCs) in the adult subventricular zone (SVZ) are associated with ependymal and vasculature niches, which regulate stem cell self-renewal and differentiation. Activated Type B stem cells and their progeny, the transit-amplifying type C cells, which express EGFR, are most highly associated with vascular cells, indicating that this niche supports lineage progression. Here, we show that proliferative SVZ progenitor cells home to endothelial cells in a stromal-derived factor 1 (SDF1)- and CXC chemokine receptor 4 (CXCR4)-dependent manner. We show that SDF1 strongly upregulates EGFR and alpha6 integrin in activated type B and type C cells, enhancing their activated state and their ability to bind laminin in the vascular niche. SDF1 increases the motility of type A neuroblasts, which migrate from the SVZ toward the olfactory bulb. Thus, differential responses to SDF1 can regulate progenitor cell occupancy of and exit from the adult SVZ vascular niche.
Copyright 2010 Elsevier Inc. All rights reserved.
Figures


Comment in
-
There's no place like home for a neural stem cell.Cell Stem Cell. 2010 Aug 6;7(2):141-3. doi: 10.1016/j.stem.2010.07.001. Cell Stem Cell. 2010. PMID: 20682440
References
-
- Alvarez-Buylla A, Lim DA. For the long run: maintaining germinal niches in the adult brain. Neuron. 2004;41:683–686. - PubMed
-
- Arvidsson A, Collin T, Kirik D, Kokaia Z, Lindvall O. Neuronal replacement from endogenous precursors in the adult brain after stroke. Nat Med. 2002;8:963–970. - PubMed
-
- Ayuso-Sacido A, Moliterno JA, Kratovac S, Kapoor GS, O'Rourke DM, Holland EC, Garcia-Verdugo JM, Roy NS, Boockvar JA. Activated EGFR signaling increases proliferation, survival, and migration and blocks neuronal differentiation in post-natal neural stem cells. J Neurooncol. 2009 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

