An emerging role for epigenetic dysregulation in arsenic toxicity and carcinogenesis
- PMID: 20682481
- PMCID: PMC3018488
- DOI: 10.1289/ehp.1002114
An emerging role for epigenetic dysregulation in arsenic toxicity and carcinogenesis
Abstract
Background: Exposure to arsenic, an established human carcinogen, through consumption of highly contaminated drinking water is a worldwide public health concern. Several mechanisms by which arsenical compounds induce tumorigenesis have been proposed, including oxidative stress, genotoxic damage, and chromosomal abnormalities. Recent studies have suggested that epigenetic mechanisms may also mediate toxicity and carcinogenicity resulting from arsenic exposure.
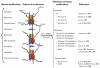
Objective: We examined the evidence supporting the roles of the three major epigenetic mechanisms-DNA methylation, histone modification, and microRNA (miRNA) expression-in arsenic toxicity and, in particular, carcinogenicity. We also investigated future research directions necessary to clarify epigenetic and other mechanisms in humans.
Data sources and synthesis: We conducted a PubMed search of arsenic exposure and epigenetic modification through April 2010 and summarized the in vitro and in vivo research findings, from both our group and others, on arsenic-associated epigenetic alteration and its potential role in toxicity and carcinogenicity.
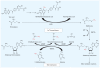
Conclusions: Arsenic exposure has been shown to alter methylation levels of both global DNA and gene promoters; histone acetylation, methylation, and phosphorylation; and miRNA expression, in studies analyzing mainly a limited number of epigenetic end points. Systematic epigenomic studies in human populations exposed to arsenic or in patients with arsenic-associated cancer have not yet been performed. Such studies would help to elucidate the relationship between arsenic exposure, epigenetic dysregulation, and carcinogenesis and are becoming feasible because of recent technological advancements.
Figures
Similar articles
-
Genotoxic and epigenetic mechanisms in arsenic carcinogenicity.Arch Toxicol. 2014 May;88(5):1043-67. doi: 10.1007/s00204-014-1233-7. Epub 2014 Apr 2. Arch Toxicol. 2014. PMID: 24691704 Review.
-
Epigenetic targets of arsenic: emphasis on epigenetic modifications during carcinogenesis.J Environ Pathol Toxicol Oncol. 2015;34(1):63-84. doi: 10.1615/jenvironpatholtoxicoloncol.2014012066. J Environ Pathol Toxicol Oncol. 2015. PMID: 25746832 Review.
-
Arsenic Exposure and Epigenetic Alterations: Recent Findings Based on the Illumina 450K DNA Methylation Array.Curr Environ Health Rep. 2015 Jun;2(2):137-44. doi: 10.1007/s40572-015-0052-1. Curr Environ Health Rep. 2015. PMID: 26231363 Free PMC article. Review.
-
Long-term arsenic exposure induces histone H3 Lys9 dimethylation without altering DNA methylation in the promoter region of p16(INK4a) and down-regulates its expression in the liver of mice.J Appl Toxicol. 2013 Sep;33(9):951-8. doi: 10.1002/jat.2765. Epub 2012 Jun 25. J Appl Toxicol. 2013. PMID: 22733434
-
Effects of arsenic toxicity beyond epigenetic modifications.Environ Geochem Health. 2018 Jun;40(3):955-965. doi: 10.1007/s10653-017-9967-9. Epub 2017 May 8. Environ Geochem Health. 2018. PMID: 28484874 Review.
Cited by
-
Agglomerates of aberrant DNA methylation are associated with toxicant-induced malignant transformation.Epigenetics. 2012 Nov;7(11):1238-48. doi: 10.4161/epi.22163. Epub 2012 Sep 13. Epigenetics. 2012. PMID: 22976526 Free PMC article.
-
Sex-specific patterns and deregulation of endocrine pathways in the gene expression profiles of Bangladeshi adults exposed to arsenic contaminated drinking water.Toxicol Appl Pharmacol. 2015 May 1;284(3):330-8. doi: 10.1016/j.taap.2015.02.025. Epub 2015 Mar 7. Toxicol Appl Pharmacol. 2015. PMID: 25759245 Free PMC article.
-
Arsenite malignantly transforms human prostate epithelial cells in vitro by gene amplification of mutated KRAS.PLoS One. 2019 Apr 22;14(4):e0215504. doi: 10.1371/journal.pone.0215504. eCollection 2019. PLoS One. 2019. PMID: 31009485 Free PMC article.
-
Mapping dynamic histone modification patterns during arsenic-induced malignant transformation of human bladder cells.Toxicol Appl Pharmacol. 2018 Sep 15;355:164-173. doi: 10.1016/j.taap.2018.06.029. Epub 2018 Jun 30. Toxicol Appl Pharmacol. 2018. PMID: 29966674 Free PMC article.
-
Arsenic Induces Members of the mmu-miR-466-669 Cluster Which Reduces NeuroD1 Expression.Toxicol Sci. 2018 Mar 1;162(1):64-78. doi: 10.1093/toxsci/kfx241. Toxicol Sci. 2018. PMID: 29121352 Free PMC article.
References
-
- Ahlborn GJ, Nelson GM, Ward WO, Knapp G, Allen JW, Ouyang M, et al. Dose response evaluation of gene expression profiles in the skin of K6/ODC mice exposed to sodium arsenite. Toxicol Appl Pharmacol. 2008;227(3):400–416. - PubMed
-
- Anetor JI, Wanibuchi H, Fukushima S. Arsenic exposure and its health effects and risk of cancer in developing countries: micronutrients as host defence. Asian Pac J Cancer Prev. 2007;8(1):13–23. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

