The C-terminal acidic tail is responsible for the inhibitory effects of HMGB1 on efferocytosis
- PMID: 20682624
- PMCID: PMC3072233
- DOI: 10.1189/jlb.0510262
The C-terminal acidic tail is responsible for the inhibitory effects of HMGB1 on efferocytosis
Abstract
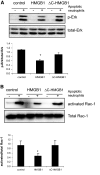
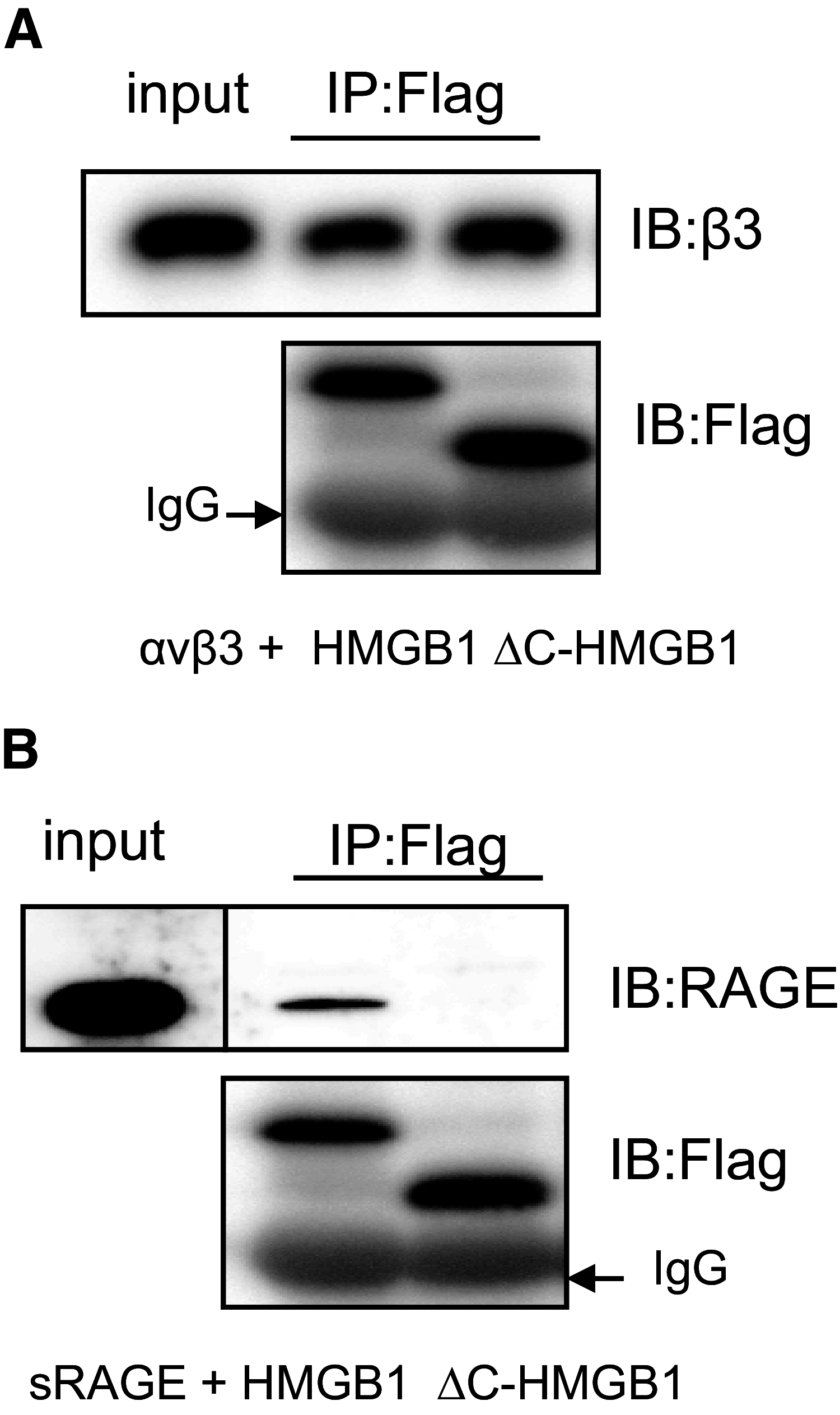
HMGB1 was described originally as a nuclear protein involved in DNA binding and transcriptional regulation. However, HMGB1 also has an extracellular role as a potent mediator of inflammation and can diminish the uptake of apoptotic cells by phagocytes, a process called efferocytosis. To explore the mechanism responsible for the ability of HMGB1 to inhibit efferocytosis, we examined the role of the C-terminal acidic tail, a region of HMGB1 that has been shown to participate in specific intramolecular interactions. Deletion of the C-terminal tail abrogated the ability of HMGB1 to decrease murine macrophage ingestion of apoptotic neutrophils and to diminish phagocytosis-induced activation of Erk and Rac-1 in macrophages. We found that RAGE plays a major role in efferocytosis, and deletion of the C-terminal tail of HMGB1 prevented binding of HMGB1 to RAGE but not to other macrophage receptors involved in efferocytosis, such as the α(V)β(3) integrin. Whereas HMGB1 decreased ingestion of apoptotic neutrophils significantly by alveolar macrophages under in vivo conditions in the lungs of mice, this effect was lost when the C-terminal acidic tail was absent from HMGB1. These results demonstrate that the HMGB1 C-terminal tail is responsible for the inhibitory effects of HMGB1 on phagocytosis of apoptotic neutrophils under in vitro and in vivo conditions.
Figures



Similar articles
-
HMGB1 inhibits macrophage activity in efferocytosis through binding to the alphavbeta3-integrin.Am J Physiol Cell Physiol. 2010 Dec;299(6):C1267-76. doi: 10.1152/ajpcell.00152.2010. Epub 2010 Sep 8. Am J Physiol Cell Physiol. 2010. PMID: 20826760 Free PMC article.
-
Poly(ADP-ribosyl)ation of high mobility group box 1 (HMGB1) protein enhances inhibition of efferocytosis.Mol Med. 2012 May 9;18(1):359-69. doi: 10.2119/molmed.2011.00203. Mol Med. 2012. PMID: 22204001 Free PMC article.
-
Urokinase-type plasminogen activator inhibits efferocytosis of neutrophils.Am J Respir Crit Care Med. 2010 Dec 15;182(12):1516-23. doi: 10.1164/rccm.201003-0452OC. Epub 2010 Jul 23. Am J Respir Crit Care Med. 2010. PMID: 20656938 Free PMC article.
-
High mobility group protein-1 inhibits phagocytosis of apoptotic neutrophils through binding to phosphatidylserine.J Immunol. 2008 Sep 15;181(6):4240-6. doi: 10.4049/jimmunol.181.6.4240. J Immunol. 2008. PMID: 18768881 Free PMC article.
-
Clearance of apoptotic neutrophils and resolution of inflammation.Immunol Rev. 2016 Sep;273(1):357-70. doi: 10.1111/imr.12453. Immunol Rev. 2016. PMID: 27558346 Free PMC article. Review.
Cited by
-
HMGB1 promotes neutrophil extracellular trap formation through interactions with Toll-like receptor 4.Am J Physiol Lung Cell Mol Physiol. 2013 Mar 1;304(5):L342-9. doi: 10.1152/ajplung.00151.2012. Epub 2013 Jan 11. Am J Physiol Lung Cell Mol Physiol. 2013. PMID: 23316068 Free PMC article.
-
Extracellular histones inhibit efferocytosis.Mol Med. 2012 Jul 18;18(1):825-33. doi: 10.2119/molmed.2012.00005. Mol Med. 2012. PMID: 22495510 Free PMC article.
-
Differential activation of RAGE by HMGB1 modulates neutrophil-associated NADPH oxidase activity and bacterial killing.Am J Physiol Cell Physiol. 2012 Jan 1;302(1):C249-56. doi: 10.1152/ajpcell.00302.2011. Epub 2011 Oct 19. Am J Physiol Cell Physiol. 2012. PMID: 22012330 Free PMC article.
-
Dietary Antioxidants Significantly Attenuate Hyperoxia-Induced Acute Inflammatory Lung Injury by Enhancing Macrophage Function via Reducing the Accumulation of Airway HMGB1.Int J Mol Sci. 2020 Feb 1;21(3):977. doi: 10.3390/ijms21030977. Int J Mol Sci. 2020. PMID: 32024151 Free PMC article.
-
Epigallocatechin-3-gallate attenuates neointimal hyperplasia in a rat model of carotid artery injury by inhibition of high mobility group box 1 expression.Exp Ther Med. 2017 Sep;14(3):1975-1982. doi: 10.3892/etm.2017.4774. Epub 2017 Jul 11. Exp Ther Med. 2017. PMID: 28962112 Free PMC article.
References
-
- Bianchi M. E., Agresti A. (2005) HMG proteins: dynamic players in gene regulation and differentiation. Curr. Opin. Genet. Dev. 15, 496–506. - PubMed
-
- Dumitriu I. E., Baruah P., Manfredi A. A., Bianchi M. E., Rovere-Querini P. (2005) HMGB1: guiding immunity from within. Trends Immunol. 26, 381–387. - PubMed
-
- Angus D. C., Yang L., Kong L., Kellum J. A., Delude R. L., Tracey K. J., Weissfeld L. (2007) Circulating high-mobility group box 1 (HMGB1) concentrations are elevated in both uncomplicated pneumonia and pneumonia with severe sepsis. Crit. Care Med. 35, 1061–1067. - PubMed
-
- Abraham E., Arcaroli J., Carmody A., Wang H., Tracey K. J. (2000) HMG-1 as a mediator of acute lung inflammation. J. Immunol. 165, 2950–2954. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

