Epidermal growth factor receptor-targeted radioimmunotherapy of human head and neck cancer xenografts using 90Y-labeled fully human antibody panitumumab
- PMID: 20682654
- PMCID: PMC3629966
- DOI: 10.1158/1535-7163.MCT-10-0444
Epidermal growth factor receptor-targeted radioimmunotherapy of human head and neck cancer xenografts using 90Y-labeled fully human antibody panitumumab
Abstract
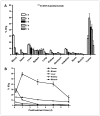
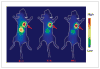
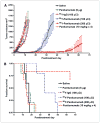
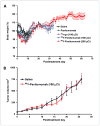
Panitumumab (ABX-EGF or Vectibix), the first fully human monoclonal antibody targeting epidermal growth factor receptor (EGFR), was approved by the Food and Drug Administration for treatment of patients with metastatic colorectal cancer. Here, we report for the first time the radioimmunotherapy (RIT) of EGFR-positive human head and neck cancer in a nude mouse model using pure beta(-) emitter (90)Y-labeled panitumumab. Biodistribution and planar gamma-imaging studies were carried out with (111)In-DOTA-panitumumab. The RIT efficacy of (90)Y-DOTA-panitumumab was evaluated in UM-SCC-22B tumor model. CD31, Ki67, terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling, and H&E staining were done on UM-SCC-22B tumor sections after treatment. The tumor uptake of (111)In-DOTA-panitumumab in UM-SCC-22B tumor-bearing nude mice was 26.10 +/- 4.93, 59.11 +/- 7.22, 44.57 +/- 9.80, 40.38 +/- 7.76, and 14.86 +/- 7.23 % injected dose per gram of tissue at 4, 24, 72, 120, and 168 hours after injection, respectively. Immunotherapy with cold panitumumab (four doses of 10 mg/kg) did not cause significant antitumor effect. RIT with a single dose of 100 microCi (90)Y-DOTA-panitumumab caused significant tumor growth delay and improved the survival in UM-SCC-22B tumor model. A single dose of 200 microCi (90)Y-DOTA-panitumumab led to almost complete tumor regression (tumor volumes were 34.83 +/- 11.11 mm(3) and 56.02 +/- 39.95 mm(3) on days 0 and 46 after treatment, respectively). Histopathologic analysis of tumors and normal organs further validated the therapeutic efficacy and limited systemic toxicity of (90)Y-DOTA-panitumumab. The high tumor uptake and prolonged tumor retention, as well as effective therapy, reveal that (90)Y-DOTA-panitumumab may be a promising radioimmunotherapeutic agent to treat EGFR-positive solid tumors.
(c) 2010 AACR.
Conflict of interest statement
No potential conflicts of interest were disclosed.
Figures


References
-
- Haddad RI, Shin DM. Recent advances in head and neck cancer. N Engl J Med. 2008;359:1143–54. - PubMed
-
- Vokes EE, Weichselbaum RR, Lippman SM, Hong WK. Head and neck cancer. N Engl J Med. 1993;328:184–94. - PubMed
-
- Alvi A, Johnson JT. Development of distant metastasis after treatment of advanced-stage head and neck cancer. Head Neck. 1997;19:500–5. - PubMed
-
- Gold KA, Lee HY, Kim ES. Targeted therapies in squamous cell carcinoma of the head and neck. Cancer. 2009;115:922–35. - PubMed
-
- Argiris A, Li Y, Forastiere A. Prognostic factors and long-term survivorship in patients with recurrent or metastatic carcinoma of the head and neck. Cancer. 2004;101:2222–9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

