Femoral adipose tissue may accumulate the fat that has been recycled as VLDL and nonesterified fatty acids
- PMID: 20682685
- PMCID: PMC3279526
- DOI: 10.2337/db10-0678
Femoral adipose tissue may accumulate the fat that has been recycled as VLDL and nonesterified fatty acids
Abstract
Objective: Gluteo-femoral, in contrast to abdominal, fat accumulation appears protective against diabetes and cardiovascular disease. Our objective was to test the hypothesis that this reflects differences in the ability of the two depots to sequester fatty acids, with gluteo-femoral fat acting as a longer-term "sink."
Research design and methods: A total of 12 healthy volunteers were studied after an overnight fast and after ingestion of a mixed meal. Blood samples were taken from veins draining subcutaneous femoral and abdominal fat and compared with arterialized blood samples. Stable isotope-labeled fatty acids were used to trace specific lipid fractions. In 36 subjects, adipose tissue blood flow in the two depots was monitored with (133)Xe.
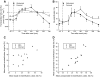
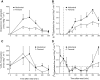
Results: Blood flow increased in response to the meal in both depots, and these responses were correlated (r(s) = 0.44, P < 0.01). Nonesterified fatty acid (NEFA) release was suppressed after the meal in both depots; it was lower in femoral fat than in abdominal fat (P < 0.01). Plasma triacylglycerol (TG) extraction by femoral fat was also lower than that by abdominal fat (P = 0.05). Isotopic tracers showed that the difference was in chylomicron-TG extraction. VLDL-TG extraction and direct NEFA uptake were similar in the two depots.
Conclusions: Femoral fat shows lower metabolic fluxes than subcutaneous abdominal fat, but differs in its relative preference for extracting fatty acids directly from the plasma NEFA and VLDL-TG pools compared with chylomicron-TG.
Figures


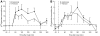
Similar articles
-
Dietary fatty acids make a rapid and substantial contribution to VLDL-triacylglycerol in the fed state.Am J Physiol Endocrinol Metab. 2007 Mar;292(3):E732-9. doi: 10.1152/ajpendo.00409.2006. Epub 2006 Nov 7. Am J Physiol Endocrinol Metab. 2007. PMID: 17090753 Clinical Trial.
-
Contributions of different fatty acid sources to very low-density lipoprotein-triacylglycerol in the fasted and fed states.J Clin Endocrinol Metab. 2006 Apr;91(4):1446-52. doi: 10.1210/jc.2005-1709. Epub 2006 Jan 31. J Clin Endocrinol Metab. 2006. PMID: 16449340 Clinical Trial.
-
Differences in partitioning of meal fatty acids into blood lipid fractions: a comparison of linoleate, oleate, and palmitate.Am J Physiol Endocrinol Metab. 2009 Jan;296(1):E64-71. doi: 10.1152/ajpendo.90730.2008. Epub 2008 Oct 21. Am J Physiol Endocrinol Metab. 2009. PMID: 18940935 Free PMC article.
-
Tracing the fate of dietary fatty acids: metabolic studies of postprandial lipaemia in human subjects.Proc Nutr Soc. 2011 Aug;70(3):342-50. doi: 10.1017/S002966511100084X. Proc Nutr Soc. 2011. PMID: 21781361 Review.
-
Structure-function of CD36 and importance of fatty acid signal transduction in fat metabolism.Annu Rev Nutr. 2014;34:281-303. doi: 10.1146/annurev-nutr-071812-161220. Epub 2014 May 16. Annu Rev Nutr. 2014. PMID: 24850384 Free PMC article. Review.
Cited by
-
Regulation of human subcutaneous adipose tissue blood flow.Int J Obes (Lond). 2014 Aug;38(8):1019-26. doi: 10.1038/ijo.2013.200. Epub 2013 Oct 29. Int J Obes (Lond). 2014. PMID: 24166067 Review.
-
Contribution of Adipose Tissue to the Chronic Immune Activation and Inflammation Associated With HIV Infection and Its Treatment.Front Immunol. 2021 Jun 18;12:670566. doi: 10.3389/fimmu.2021.670566. eCollection 2021. Front Immunol. 2021. PMID: 34220817 Free PMC article. Review.
-
Hyperinsulinaemia: does it tip the balance toward intrahepatic fat accumulation?Endocr Connect. 2019 Oct;8(10):R157-R168. doi: 10.1530/EC-19-0350. Endocr Connect. 2019. PMID: 31581129 Free PMC article. Review.
-
CD73-dependent generation of extracellular adenosine by vascular endothelial cells modulates de novo lipogenesis in adipose tissue.Front Immunol. 2024 Jan 9;14:1308456. doi: 10.3389/fimmu.2023.1308456. eCollection 2023. Front Immunol. 2024. PMID: 38264660 Free PMC article.
-
Does Food Affect the Pharmacokinetics of Non-orally Delivered Drugs? A Review of Currently Available Evidence.AAPS J. 2022 Apr 29;24(3):59. doi: 10.1208/s12248-022-00714-0. AAPS J. 2022. PMID: 35488003 Review.
References
-
- Yusuf S, Hawken S, Ounpuu S, Bautista L, Franzosi MG, Commerford P, Lang CC, Rumboldt Z, Onen CL, Lisheng L, Tanomsup S, Wangai P, Jr, Razak F, Sharma AM, Anand SS. INTERHEART Study Investigators Obesity and the risk of myocardial infarction in 27,000 participants from 52 countries: a case-control study. Lancet 2005;366:1640–1649 - PubMed
-
- Pischon T, Boeing H, Hoffmann K, Bergmann M, Schulze MB, Overvad K, van der Schouw YT, Spencer E, Moons KG, Tjønneland A, Halkjaer J, Jensen MK, Stegger J, Clavel-Chapelon F, Boutron-Ruault MC, Chajes V, Linseisen J, Kaaks R, Trichopoulou A, Trichopoulos D, Bamia C, Sieri S, Palli D, Tumino R, Vineis P, Panico S, Peeters PH, May AM, Bueno-de-Mesquita HB, van Duijnhoven FJ, Hallmans G, Weinehall L, Manjer J, Hedblad B, Lund E, Agudo A, Arriola L, Barricarte A, Navarro C, Martinez C, Quirós JR, Key T, Bingham S, Khaw KT, Boffetta P, Jenab M, Ferrari P, Riboli E. General and abdominal adiposity and risk of death in Europe. N Engl J Med 2008;359:2105–2120 - PubMed
-
- Manolopoulos KN, Karpe F, Frayn KN. Gluteofemoral body fat as a determinant of metabolic health. Int J Obes 2010;34:949–959 - PubMed
-
- Rebuffé-Scrive M, Lönnroth P, Mårin P, Wesslau C, Björntorp P, Smith U. Regional adipose tissue metabolism in men and postmenopausal women. Int J Obes 1987;11:347–355 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

