Hypothalamic AMP-activated protein kinase regulates glucose production
- PMID: 20682691
- PMCID: PMC3279556
- DOI: 10.2337/db10-0221
Hypothalamic AMP-activated protein kinase regulates glucose production
Abstract
Objective: The fuel sensor AMP-activated protein kinase (AMPK) in the hypothalamus regulates energy homeostasis by sensing nutritional and hormonal signals. However, the role of hypothalamic AMPK in glucose production regulation remains to be elucidated. We hypothesize that bidirectional changes in hypothalamic AMPK activity alter glucose production.
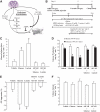
Research design and methods: To introduce bidirectional changes in hypothalamic AMPK activity in vivo, we first knocked down hypothalamic AMPK activity in male Sprague-Dawley rats by either injecting an adenovirus expressing the dominant-negative form of AMPK (Ad-DN AMPKα2 [D(157)A]) or infusing AMPK inhibitor compound C directly into the mediobasal hypothalamus. Next, we independently activated hypothalamic AMPK by delivering either an adenovirus expressing the constitutive active form of AMPK (Ad-CA AMPKα1(312) [T172D]) or the AMPK activator AICAR. The pancreatic (basal insulin)-euglycemic clamp technique in combination with the tracer-dilution methodology was used to assess the impact of alternations in hypothalamic AMPK activity on changes in glucose kinetics in vivo.
Results: Injection of Ad-DN AMPK into the hypothalamus knocked down hypothalamic AMPK activity and led to a significant suppression of glucose production with no changes in peripheral glucose uptake during the clamps. In parallel, hypothalamic infusion of AMPK inhibitor compound C lowered glucose production as well. Conversely, molecular and pharmacological activation of hypothalamic AMPK negated the ability of hypothalamic nutrients to lower glucose production.
Conclusions: These data indicate that changes in hypothalamic AMPK activity are sufficient and necessary for hypothalamic nutrient-sensing mechanisms to alter glucose production in vivo.
Figures

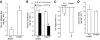

References
-
- Kahn BB, Alquier T, Carling D, Hardie DG. AMP-activated protein kinase: ancient energy gauge provides clues to modern understanding of metabolism. Cell Metab 2005;1:15–25 - PubMed
-
- McGarry JD. Banting lecture 2001: dysregulation of fatty acid metabolism in the etiology of type 2 diabetes. Diabetes 2002;51:7–18 - PubMed
-
- Lam TK, Schwartz GJ, Rossetti L. Hypothalamic sensing of fatty acids. Nat Neurosci 2005;8:579–584 - PubMed
-
- Kubota N, Yano W, Kubota T, Yamauchi T, Itoh S, Kumagai H, Kozono H, Takamoto I, Okamoto S, Shiuchi T, Suzuki R, Satoh H, Tsuchida A, Moroi M, Sugi K, Noda T, Ebinuma H, Ueta Y, Kondo T, Araki E, Ezaki O, Nagai R, Tobe K, Terauchi Y, Ueki K, Minokoshi Y, Kadowaki T. Adiponectin stimulates AMP-activated protein kinase in the hypothalamus and increases food intake. Cell Metab 2007;6:55–68 - PubMed
-
- Kola B, Hubina E, Tucci SA, Kirkham TC, Garcia EA, Mitchell SE, Williams LM, Hawley SA, Hardie DG, Grossman AB, Korbonits M. Cannabinoids and ghrelin have both central and peripheral metabolic and cardiac effects via AMP-activated protein kinase. J Biol Chem 2005;280:25196–25201 - PubMed

