Increased reactive oxygen species production and lower abundance of complex I subunits and carnitine palmitoyltransferase 1B protein despite normal mitochondrial respiration in insulin-resistant human skeletal muscle
- PMID: 20682693
- PMCID: PMC3279558
- DOI: 10.2337/db10-0174
Increased reactive oxygen species production and lower abundance of complex I subunits and carnitine palmitoyltransferase 1B protein despite normal mitochondrial respiration in insulin-resistant human skeletal muscle
Abstract
Objective: The contribution of mitochondrial dysfunction to skeletal muscle insulin resistance remains elusive. Comparative proteomics are being applied to generate new hypotheses in human biology and were applied here to isolated mitochondria to identify novel changes in mitochondrial protein abundance present in insulin-resistant muscle.
Research design and methods: Mitochondria were isolated from vastus lateralis muscle from lean and insulin-sensitive individuals and from obese and insulin-resistant individuals who were otherwise healthy. Respiration and reactive oxygen species (ROS) production rates were measured in vitro. Relative abundances of proteins detected by mass spectrometry were determined using a normalized spectral abundance factor method.
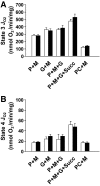
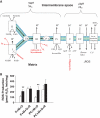
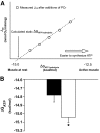
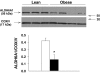
Results: NADH- and FADH(2)-linked maximal respiration rates were similar between lean and obese individuals. Rates of pyruvate and palmitoyl-DL-carnitine (both including malate) ROS production were significantly higher in obesity. Mitochondria from obese individuals maintained higher (more negative) extramitochondrial ATP free energy at low metabolic flux, suggesting that stronger mitochondrial thermodynamic driving forces may underlie the higher ROS production. Tandem mass spectrometry identified protein abundance differences per mitochondrial mass in insulin resistance, including lower abundance of complex I subunits and enzymes involved in the oxidation of branched-chain amino acids (BCAA) and fatty acids (e.g., carnitine palmitoyltransferase 1B).
Conclusions: We provide data suggesting normal oxidative capacity of mitochondria in insulin-resistant skeletal muscle in parallel with high rates of ROS production. Furthermore, we show specific abundance differences in proteins involved in fat and BCAA oxidation that might contribute to the accumulation of lipid and BCAA frequently associated with the pathogenesis of insulin resistance.
Figures
References
-
- Szendroedi J, Roden M. Ectopic lipids and organ function. Curr Opin Lipidol 2009;20:50–56 - PubMed
-
- Vondra K, Rath R, Bass A, Slabochova Z, Teisinger J, Vitek V. Enzyme activities in quadriceps femoris muscle of obese diabetic male patients. Diabetologia 1977;13:527–529 - PubMed
-
- Morino K, Petersen KF, Dufour S, Befroy D, Frattini J, Shatzkes N, Neschen S, White MF, Bilz S, Sono S, Pypaert M, Shulman GI. Reduced mitochondrial density and increased IRS-1 serine phosphorylation in muscle of insulin-resistant offspring of type 2 diabetic parents. J Clin Invest 2005;115:3587–3593 - PMC - PubMed
-
- Kelley DE, He J, Menshikova EV, Ritov VB. Dysfunction of mitochondria in human skeletal muscle in type 2 diabetes. Diabetes 2002;51:2944–2950 - PubMed
-
- Ritov VB, Menshikova EV, He J, Ferrell RE, Goodpaster BH, Kelley DE. Deficiency of subsarcolemmal mitochondria in obesity and type 2 diabetes. Diabetes 2005;54:8–14 - PubMed

