T-type channels control the opioidergic descending analgesia at the low threshold-spiking GABAergic neurons in the periaqueductal gray
- PMID: 20682748
- PMCID: PMC2930412
- DOI: 10.1073/pnas.1009532107
T-type channels control the opioidergic descending analgesia at the low threshold-spiking GABAergic neurons in the periaqueductal gray
Abstract
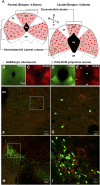
Endogenous opioids generate analgesic signals in the periaqueductal gray (PAG). However, because cell types in the PAG are difficult to identify, its neuronal mechanism has remained poorly understood. To address this issue, we characterized PAG neurons by their electrical properties using differentially labeled GABAergic and output neurons in the PAG. We found that GABAergic neurons were mostly fast-spiking cells and could be further divided into two distinct classes: with or without low-threshold spikes (LTS) driven by T-type channels. In contrast, the PAG output neurons lacked LTS and showed heterogeneous firing patterns. To reveal the function of the LTS, we examined the mutant mice lacking the alpha1G T-type channels (alpha1G(-/-)). The mutant mice lacked LTS in the fast-spiking GABAergic neurons of the PAG and unexpectedly showed impaired opioid-dependent analgesia; a similar phenotype was reproduced in PAG-specific alpha1G-knockdown mice. Electrophysiological analyses revealed functional expression of mu-opioid receptors in the low threshold-spiking GABAergic neurons. These neurons in the mutant lacking LTS showed markedly enhanced discharge activities, which led to an augmented inhibition of output neurons. Furthermore, the impaired analgesia observed in alpha1G(-/-) mice was reversed by blocking local GABA(A) receptors. These results indicate that alpha1G T-type channels are critical for the opioidergic descending analgesia system in the PAG.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


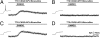


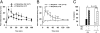

References
-
- Yaksh TL, Yeung JC, Rudy TA. Systematic examination in the rat of brain sites sensitive to the direct application of morphine: Observation of differential effects within the periaqueductal gray. Brain Res. 1976;114:83–103. - PubMed
-
- Reichling DB, Kwiat GC, Basbaum AI. Anatomy, physiology and pharmacology of the periaqueductal gray contribution to antinociceptive controls. Prog Brain Res. 1988;77:31–46. - PubMed
-
- Dostrovsky JO, Deakin JFW. Periaqueductal grey lesions reduce morphine analgesia in the rat. Neurosci Lett. 1977;4:99–103. - PubMed
-
- Lewis VA, Gebhart GF. Evaluation of the periaqueductal central gray (PAG) as a morphine-specific locus of action and examination of morphine-induced and stimulation-produced analgesia at coincident PAG loci. Brain Res. 1977;124:283–303. - PubMed
-
- Basbaum AI, Fields HL. Endogenous pain control systems: Brainstem spinal pathways and endorphin circuitry. Annu Rev Neurosci. 1984;7:309–338. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

